The virus that causes COVID-19 has been very good at mutating to keep infecting people – so good that most antibody treatments developed during the pandemic are no longer effective. Now a team led by Stanford University researchers may have found a way to pin down the constantly evolving virus and develop longer-lasting treatments.
The researchers discovered a method to use two antibodies, one to serve as a type of anchor by attaching to an area of the virus that does not change very much and another to inhibit the virus’s ability to infect cells. This pairing of antibodies was shown to be effective against the initial SARS-CoV-2 virus that caused the pandemic and all its variants through omicron in laboratory testing. The findings are detailed in the journal Science Translational Medicine.
“In the face of an ever-changing virus, we engineered a new generation of therapeutics that have the ability to be resistant to viral evolution, which could be useful many years down the road for the treatment of people infected with SARS-CoV-2,” said Christopher O. Barnes, the study’s senior author, an assistant professor of biology in the Stanford School of Humanities and Sciences and a scholar at Stanford’s Sarafan CheM-H.
An overlooked option
The team led by Barnes and first author Adonis Rubio, a doctoral candidate in the Stanford School of Medicine, conducted this investigation using donated antibodies from patients who had recovered from COVID-19. Analyzing how these antibodies interacted with the virus, they found one that attaches to a region of the virus that does not mutate often.
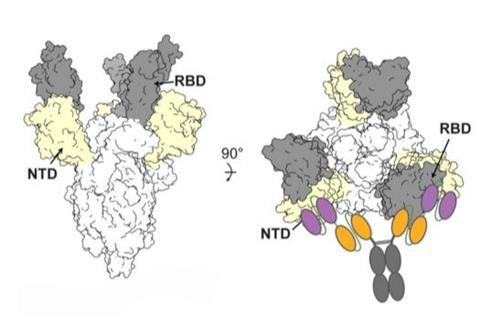
This area, within the Spike N-terminal domain, or NTD, had been overlooked because it was not directly useful for treatment. However, when a specific antibody attaches to this area, it remains stuck to the virus. This is useful when designing new therapies that enable another type of antibody to get a foothold and attach to the receptor-binding domain, or RBD, of the virus, essentially blocking the virus from binding to receptors in human cells.
READ MORE: New antibody breakthrough offers hope against evolving SARS-CoV-2 variants
READ MORE: Mutations of the spike gene do not predict the severity of variants of SARS-CoV-2
The researchers designed a series of these dual or “bispecific” antibodies, called CoV2-biRN, and in laboratory tests they showed high neutralization of all the variants of SARS-CoV-2 known to cause illness in humans. The antibodies also significantly reduced the viral load in the lungs of mice exposed to one version of the omicron variant.
More research, including clinical trials, would have to be done before this discovery could be used as a treatment in human patients, but the approach is promising – and not just for the virus that causes COVID-19.
Wide applications
Next, the researchers will work to design bispecific antibodies that would be effective against all coronaviruses, the virus family including the ones that cause the common cold, MERS, and COVID-19. This approach could potentially also be effective against influenza and HIV, the authors said.
“Viruses constantly evolve to maintain the ability to infect the population,” Barnes said. “To counter this, the antibodies we develop must continuously evolve as well to remain effective.”
Topics
- Adonis Rubio
- Bioengineering
- Christopher O. Barnes
- CoV2-biRN
- COVID-19
- Disease Treatment & Prevention
- Early Career Research
- Healthy Land
- One Health
- receptor-binding domain
- Research News
- Sarafan CheM-H
- Spike N-terminal domain
- Stanford School of Humanities and Sciences
- Stanford School of Medicine
- Stanford University
- USA & Canada
- Vaccinology
- Viruses







No comments yet