A group of Brazilian researchers has found a potential target for novel therapeutic strategies to combat fungal infections – more specifically, those caused by Aspergillus fumigatus. An article reporting the discovery is published in the journal Communications Biology.
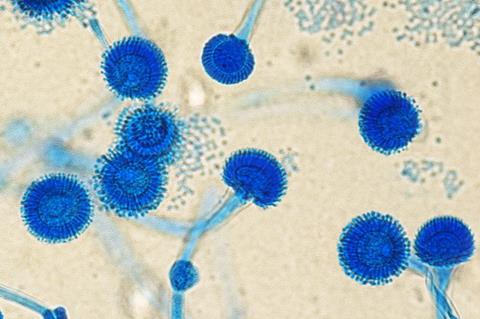
This species and other Aspergillus fungi are responsible for complications such as invasive pulmonary aspergillosis (IPA), a potentially fatal infection that can only occur in patients with weakened immune systems, such as those hospitalized in intensive care units (ICUs). Depending on the patient’s condition, the mortality rate from IPA ranges from 20% to 72%.
READ MORE: Fungi elude antifungal treatments by restructuring cell walls
READ MORE: Scientists unlock the secrets of disease-causing fungus Aspergillus fumigatus
“We found that silencing a specific gene attenuated virulence. When it was deleted, the fungus remained viable, but important biological processes were affected, making the fungus less virulent and increasing the likelihood of its being eliminated by existing medications or even by the human immune system,” said Natália Sayuri Wassano, first author of the article. The study was conducted while she was a PhD candidate at the State University of Campinas’s Biology Institute (IB-UNICAMP) with a scholarship from FAPESP, and was part of a project supported by FAPESP.
Adverse side effects
Resistance to existing antifungals is frequent, and chronic IAP patients may spend years undergoing treatment. The available therapies can also have adverse side-effects, such as vomiting and headache.
“Very few new antifungal drugs have been approved for use in recent years. Molecules that can have complementary action with others already in use, however, have shown significant potential and are highly likely to be approved,” said André Damasio, co-corresponding author of the article and a professor at IB-UNICAMP.
The other corresponding author is Nilmar Moretti, a professor at the Federal University of São Paulo’s Medical School (EPM-UNIFESP). He and Damasio belong to the National Institute of Science and Technology (NIST) in Human Pathogenic Fungi, one of several such institutes in São Paulo State funded by the National Council for Scientific and Technological Development (CNPq) in partnership with FAPESP.
Another research group supported by FAPESP recently patented a novel compound that combats drug-resistant fungi when combined with commercially available antifungals. Two of the researchers involved in that study are co-authors of this article (more at: https://agencia.fapesp.br/41848).
Novel target
Next steps for the research group include finding a molecule that inactivates sirtuin E, the protein that produced the best results among six tested. If they find one, it could serve as a basis for a drug that would make the fungus more sensitive to existing medications.
Sirtuins, the class of proteins analyzed in the study, are also present in humans, playing a part in several cellular and physiological processes. The researchers found that 25% of the sirtuins studied were identical in the fungus and in humans; the low percentage means sirtuin could be a safe drug target.
“It isn’t necessarily bad for a future drug with this target to inhibit human sirtuin as well as fungal sirtuin, but it’s something we should look out for. Marginal side-effects can be acceptable in novel drugs, provided the benefits are substantial,” Damasio said.
Common ancestor
One of the difficulties of finding new antifungals is precisely the similarity between some of the proteins found in fungi and humans. The reason is that we are closer to fungi in evolutionary terms than we are to bacteria, for example. Genes from some common ancestor have been conserved in both humans and fungi. This partly explains why it has been possible to produce so many drugs to combat bacteria.
To arrive at their results, the researchers bred seven mutant strains of A. fumigatus, in one of which six sirtuins were deleted, while a different sirtuin was inactivated in each of the others by means of the CRISPR-Cas9 gene editing technique.
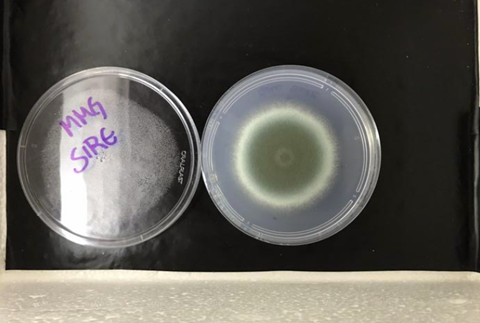
Cultured on plates in the laboratory, the mutant that did not produce any of the six sirtuins and the mutant without the gene sirE (which produces the protein sirtuin E), grew less than the wild strain at 37 °C, the average normal temperature of the human body.
The researchers then infected mice with the wild strain and the mutant without sirE. The mice infected with the mutant fungus did not develop the disease, whereas the mice infected with the wild strain died after six days.
More virulent
“We were unable to determine exactly what made this strain more virulent, but the analyses showed several possibilities. The gene in question appears to regulate key functions such as cell wall integrity and production of secondary metabolites that help defend it from the human immune system, among other things,” said Wassano, who conducted part of the experiments during a research internship at the University of Wisconsin Madison in the United States.
Wassano and Damasio are co-authors of another article about the mutant that does not produce SirE, reporting a study led by the group at UW Madison.
The new antifungal options on the market and the emergence of drug-resistant strains led the World Health Organization (WHO) to list A. fumigatus as a fungal priority pathogen to guide research, development and public health action.
“In vitro and in vivo synergy studies would allow optimization of treatment regimes”, according to the 2022 document.
The study was also supported by FAPESP via three other projects (18/09948-0, 22/02992-0 and 22/03075-0).







No comments yet