A fungus discovered in the mouse stomach may hold a key to fungal evolution within the gastrointestinal tract, according to new research led by Weill Cornell Medicine investigators. The finding suggests that preclinical studies until now have overlooked a major influencer of mouse physiology.
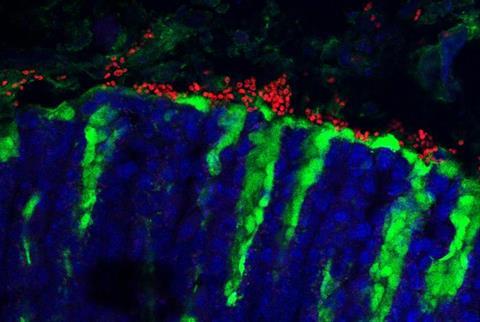
Scientists recently have come to appreciate the importance, for human health and disease, of microbes—often called “commensals”—that naturally dwell in the gut. Bacterial commensals, for example, are known to have a big influence on human immunity; abnormal changes in these populations have been tied to cancers, inflammatory disorders and even depression. However, how gut fungal commensals affect immunity is less well understood, due in part to the lack of a good mouse model of fungal commensalism.
The new study, reported Nov. 27 in Nature, found that a yeast called Kazachstania pintolopesii, abundant in the stomach of wild mice, is exceptionally well adapted to mice and benefits them by boosting their immune protection against parasites, though it also enhances their vulnerability to some allergies.
Fungal commensal
“For years we’ve been looking for a true fungal commensal in mice, but fungal populations in lab mice, as identified by analyzing fungal DNA, tend to be transient and vary greatly from colony to colony,” said study senior author Dr. Iliyan Iliev, an associate professor of immunology in medicine, member of the Jill Roberts Institute for Research in Inflammatory Bowel Disease, and a faculty member in the Immunology & Microbial Pathogenesis Graduate School Program at Weill Cornell Medicine.
READ MORE: Temper the hype of human microbiome studies
READ MORE: Gut hormone doubles as immune regulator for the fungal microbiome
In 2019, a team led by co-author Dr. Barbara Rehermann of the National Institutes of Health found that “wildling” lab mice raised with gut microbes like those of wild mice do a better job of modeling human immune responses than traditional lab mice. Dr. Iliev’s lab, which participated in that study, found significantly higher levels of fungal DNA in the gut of these mice—magnitudes greater than previously observed in lab mice.
“This was the start of a kind of Sherlock Holmes story as we went looking for the dominant fungus, extending our study to other mouse populations,” Dr. Iliev said. “And what better place to find wild mice than New York City!”
Mice in the big city
The team looked for evidence of the fungus in fecal samples and other material provided by pest-control companies in New York City and Los Angeles, and acquired samples from multiple research institutions that use or sell lab mice. Ultimately, they determined that K. pintolopesii is very common in wild mice, but also often present in lab mouse colonies without researchers knowing about its presence.
“The presence or absence of this fungus should be taken into account in many types of mouse studies,” said co-first author Dr. Yun Liao, a postdoctoral researcher in the Iliev laboratory.
“K. pintolopesii can completely change the experimental outcome,” said co-first author Dr. Iris Gao, who was a graduate student in the Iliev lab during the study.
Adapted to mice
The researchers found that K. pintolopesii can rapidly colonize the gastrointestinal tracts of laboratory mice, is reliably transmitted to mouse newborns, and somehow evades its hosts antifungal immunity even as it partially suppresses the growth of other fungal species—all of which hints that this fungus is evolutionarily adapted for living in mice and is a true commensal.
However, upon gastrointestinal mucus fluctuations caused by dietary changes or antibiotics, for example, the fungus becomes visible to the immune system by activating the production of a cytokine called IL-33. This cytokine, in turn, triggers what is known as a “type 2” immune response. The fungus symbiotically benefits its hosts by suppressing other fungi and by protecting them against worms through this enhanced type 2 immune response, but on the flip side it exacerbates food allergies, the team discovered.
“If you’re using mice to research allergies, parasite infections, cancer development, or any other area where type 2 or type 17 immune responses are relevant, then this fungus may be an important factor that you shouldn’t omit,” Dr. Iliev said.
More questions
While the study suggests that K. pintolopesii is a good model for fungal commensalism, it also raises important questions: Is this fungus a normal component of the mouse microbiota that should always be present in lab mice, especially for studies touching on immunology? Is there a fungal commensal that has a similar role in promoting type 2 immunity in humans?
Dr. Iliev and his lab are now seeking answers to these questions in samples collected across the continent in a research collaboration formed between labs in multiple institutions including the Broad Institute, The National Institutes of Health and Penn State.





No comments yet