How malaria parasites evolved to evade a major antimalarial drug has long been thought to involve only one key gene. Now, thanks to a combination of field and lab studies, an international research team has shown a second key gene is also involved in malaria’s resistance to the drug chloroquine.
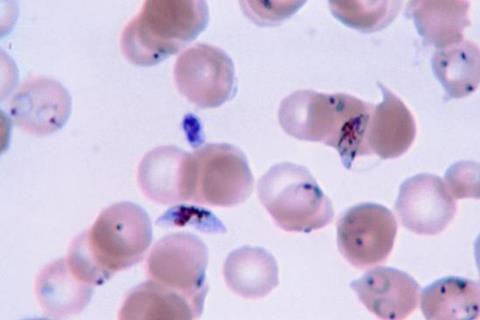
The finding, published this week in the journal Nature Microbiology, has implications for the ongoing battle against malaria, which infects an estimated 247 million people and kills more than 619,000 annually – mostly young children.
“With drug-resistant pathogens on the rise, it is important to understand how treatments drive parasite evolution and how this evolution can vary in different parts of the world,” says Texas Biomedical Research Institute Professor Timothy J.C. Anderson, PhD, and one of the lead authors of the paper.
Drug resistance
Chloroquine was developed to treat malaria in the 1950s and administered widely. Drug resistance emerged within a few years, spreading first through Southeast Asia and then through Africa in the 1970s and 80s.
Chloroquine was replaced with a succession of other antimalarial drugs, but resistance evolution remains a challenge to control the parasites. In 2000, researchers identified a gene, the chloroquine resistance transporter (pfcrt), that evolved to help the parasites transport chloroquine out of a key region of their cells, rendering the drug ineffective.
“That resistance gene, pfcrt, is infamous,” says University of Notre Dame Professor Michael Ferdig, PhD, and one of the lead paper authors. “To find that pfcrt has a partner in crime should not be a surprise – genes interact with each other as part of evolution all the time. But it was only with new tools and our integrated approach that we could finally pinpoint the specific culprit.”
Six species
Six species of malaria parasites infect humans; Plasmodium falciparum is considered the deadliest. In this paper, researchers from the Medical Research Council Unit The Gambia at the London School of Hygiene & Tropical Medicine and collaborators analyzed more than 600 P. falciparum genomes collected in The Gambia from 1984 to 2014. The 30-year dataset revealed mutations in a second gene encoding an amino acid transporter (AAT1) increased from 0% frequency in 1984 to 97% frequency in 2014.
“This is a very clear example of natural selection in action – these mutations were preferred and passed on with extremely high frequency in a very short amount of time, suggesting they provide a significant survival advantage,” says Medical Research Council Unit The Gambia at London School of Hygiene & Tropical Medicine Professor Alfred Amambua-Ngwa, PhD, and one of the first authors. “The mutations in AAT1 very closely mirror the increase of pfcrt mutations. Given this, it strongly suggests AAT1 is involved in chloroquine resistance.”
Teams at Texas Biomed, University of Notre Dame and Seattle Children’s Research Institute collaborated to evaluate experimentally how the mutations affect drug resistance. Specifically, researchers conducted genetic crosses between chloroquine-sensitive and chloroquine-resistant parasites, which suggested involvement of AAT1 mutations.
Gene-editing
Using CRISPR gene-editing technology, researchers replaced the mutations in parasite genomes in the laboratory and observed this impacted drug resistance. Collaborators at Nottingham University tested the gene’s function in yeast, which also showed that the mutations resulted in drug resistance. Collaborating institutes also included Wellcome Sanger Institute, Mahidol-Oxford Tropical Medicine Research Unit and UT Health San Antonio.
“This project would not have been possible without the dedication of multiple collaborators in the US, Europe, Asia and Africa,” says Ashley Vaughan, PhD, principal investigator at Seattle Children’s Research Institute, and one of the lead paper authors. “We brought together very diverse methodologies, which all came to the same conclusion.”
But the team did not stop there. Additional malaria genome datasets showed that the AAT1 mutations that confer resistance disappeared in Africa once chloroquine was no longer used there, which is typically expected. However, it is a completely different story in Southeast Asia where the mutations remain.
“Our analyses showed that parasites from Africa and Asia carry different pfaat1 mutations, and our experimental data suggest that this may underlie the differences we observe in drug resistance evolution in Africa and Asia,” says Dr. Ferdig.
Remarkably, researchers analyzing different malaria species infecting rodents found the same gene to be involved in chloroquine resistance more than a decade ago. “This shows me that the rodent and human malaria researchers need to be talking more,” Dr. Anderson says.
London School of Hygiene & Tropical Medicine Professor David Conway, PhD, stresses that grappling with drug resistance – for malaria and other pathogens – requires taking a holistic approach to both drug development and pathogen surveillance. “We must be aware that different genes and molecules will be working together to survive treatments,” he says. “That’s why looking at whole genomes and whole populations is so critical.”
Topics
- Alfred Amambua-Ngwa
- Asia & Oceania
- chloroquine
- David Conway
- Disease Treatment & Prevention
- Infection Prevention & Control
- London School of Hygiene & Tropical Medicine
- Mahidol-Oxford Tropical Medicine Research Unit
- Michael Ferdig
- Microbial Genomics
- Middle East & Africa
- Nottingham University
- One Health
- Parasites
- Plasmodium falciparum
- Research News
- Texas Biomedical Research Institute
- Timothy J.C. Anderson
- USA & Canada
- UT Health San Antonio
- Wellcome Sanger Institute






No comments yet