In the face of rising concerns about antibiotic resistant infections, an international group of microbial experts have launched a powerful and flexible free online genomic toolkit for more rapid development of phage therapy.
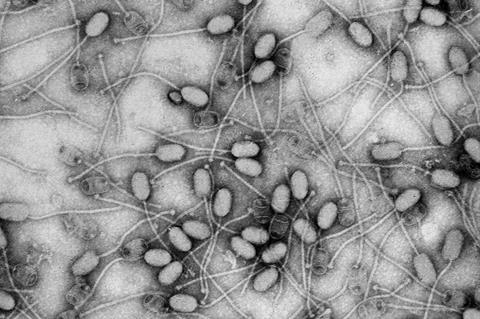
After decades of research, phages or bacteriophage viruses that target and kill specific bacteria are seen as the next frontier in finding fast and effective ways to curb the death toll and serious illnesses caused by antibiotic resistant ‘superbugs’ every year.
The lead developers at Flinders University claim the new platform, called Sphae, is capable of assessing if a phage is suitable for a targeted therapy in under 10 minutes.
This marks a big step forward in quickly evaluating phage safety and suitability for addressing antibiotic-resistant infections, according to the team at Flinders Accelerator for Microbiome Exploration (FAME) and collaborators in a new article just published in the Oxford Academic journal Bioinformatics Advances.
Vast and complex datasets
“Sphae integrates high-throughput sequencing technologies with advanced computational pipelines, enabling researchers to analyse vast and complex datasets efficiently,” says Bhavya Papudeshi, from the FAME research group at Flinders University’s College of Science and Engineering.
“Sphae prioritises safety, flagging genes associated with toxins or undesirable traits to ensure that only the safest candidates are advanced for therapeutic use,” she says.
“Adaptability and scalability sets Sphae apart. The workflow supports a wide range of sequencing technologies while the toolkit can handle the massive datasets typical of high-performance computing environments, making it an invaluable tool for labs tackling large-scale projects.”
Multiple phage genomes
Sphae not only aids in therapeutic research but also advances our broader understanding of microbial ecosystems and their impact on global health and climate, adds FAME group co-director Professor Robert Edwards, from the College of Science and Engineering at Flinders University.
“Sphae processes multiple phage genomes at once, saving time and efficiently handling larger datasets,” says Professor Edwards, Matthew Flinders Professor of Bioinformatics.
“We see Sphae works effectively even in mixed or challenging datasets, providing consistent and accurate results to help identify phages that can potentially combat resistant bacterial strains.
“It offers a complete view of phage genomes, summarising key features like resistance and virulence markers for better insight into phage safety and functionality.”
Global push against AMR
The United Nations and World Health Organization warn that antibiotic resistant infections are rising, particularly among older and vulnerable people. A recent global study published in The Lancet forecasts that potential deaths from antibiotic resistance will continue to climb and more than double to 2 million a year, with the death toll mounting to more than 39 million people by 2050, unless measures are taken urgently to find alternatives. Another 2022 study estimated that almost 5 million deaths per year are associated with drug-resistant bacteria, with a higher burden among low-income and middle-income countries.
READ MORE: A new tool to predict the most effective phage cocktail
READ MORE: New phage editing technology could lead to alternative treatments for antibiotic-resistant bacteria
Professor Edwards says initiatives to build phage banks for common pathogens such as Achromobacter, Acinetobacter, and Stenotrophomonas are part of a global push to scale up research into new antibacterial treatments.
Personalised phage therapy
“When conventional antibiotics are not effective any more, personalised phage therapy could become a standard part of medical practice by simplifying and accelerating the discovery of therapeutic phages suited to the individual patient’s infection,” says Professor Edwards.
“With programs like Phage Australia and innovations like Sphae, researchers are one step closer to unlocking the full potential of these microbial marvels.
“The future of medicine lies in the precise, efficient, and safe use of phages to combat bacterial infections and restore hope to patients worldwide.”
Topics
- Achromobacter
- Acinetobacter
- Antimicrobial Resistance
- Asia & Oceania
- Bacteria
- Bhavya Papudeshi
- Bioinformatics
- Clinical & Diagnostics
- Disease Treatment & Prevention
- Flinders University
- genomic toolkit
- Infection Prevention & Control
- Innovation News
- Microbial Genomics
- One Health
- Phage Australia
- phage banks
- Phage Therapy
- Rapid Diagnostics
- Robert Edwards
- Sphae
- Stenotrophomonas
- Viruses




No comments yet