Vanderbilt University Medical Center has joined an extraordinary national effort to develop and stockpile new vaccines and antibody therapies against a host of viral threats to prevent another pandemic.
Pending the availability of funds, the National Institute of Allergy and Infectious Disease of the National Institutes of Health expects to commit approximately $100 million a year to the program, called ReVAMPP, for Research and Development of Vaccines and Monoclonal Antibodies for Pandemic Preparedness, the NIH announced on Sept. 13.
READ MORE: Study reveals clues to how Eastern equine encephalitis virus invades brain cells
READ MORE: Newly isolated antibodies may aid effort to fight influenza B
Developed in the wake of the COVID-19 pandemic, which killed millions of people worldwide and cost the United States economy trillions of dollars, ReVAMPP will support a coordinating center and seven research centers, including VUMC, with a focus on nine virus groups that pose the greatest risk to human health.
Families of viruses
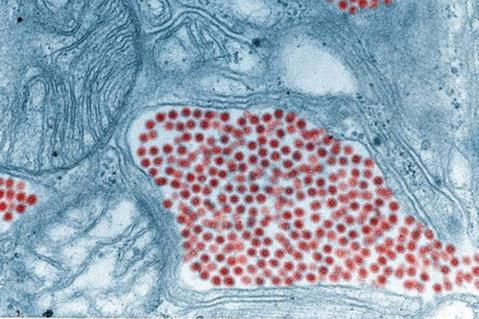
VUMC’s three-year, $13 million per year grant (number U19AI181979) will enable researchers at the Vanderbilt Vaccine Center (VVC) to accelerate their investigations of bunyaviruses, which include life-threatening respiratory and hemorrhagic fever viruses, and picornaviruses, notably enterovirus D68.
They also will contribute to ReVAMPP projects led by the University of Texas Medical Branch in Galveston and Washington University School of Medicine in St. Louis targeting other viral threats, including mosquito-transmitted Eastern equine encephalitis, which shut down parks and beaches on the East Coast this summer.
“We have long stated that antibodies and vaccines for emerging infections should be generated and tested prior to large outbreaks, not after disaster has occurred,” said James Crowe Jr., MD, director of the VVC and the grant’s principal investigator.
“This new federal program gives us the support to prepare ahead of time for prevention and treatment of some of the most lethal infections that threaten to cause human epidemics,” he said.
Evolving rapidly
Like SARS-CoV-2, a member of the coronavirus family that causes COVID-19, hundreds of viruses known to cause disease in humans evolve rapidly, making it difficult to develop long-lasting methods to contain them.
To meet this challenge, ReVAMPP is focusing on “prototype pathogens,” representatives of each virus group. Researchers will look for “sites of vulnerability” in the prototypes that can inform development of antibody therapies and vaccines capable of blocking every group member.
Through its new Bunyavirus and Picornavirus Pandemic Pathogen Preparedness (BP4) Center, the VVC will assemble a consortium of 20 leading investigators in picornavirus and bunyavirus virology, immunology, vaccine biology and antibody sciences from universities, research institutes and industry partners across the country.
Housed in a state-of-the-art lab atop a medical research tower on the Main cCampus, the VVC is uniquely prepared to contribute to ReVAMPP, said the center’s associate director, Robert Carnahan, PhD, professor of Pediatrics and of Radiology and Radiological Sciences.
White blood cells
VVC researchers have developed techniques for rapidly isolating clones of white blood cells that make antibodies targeting viral proteins. Once identified, these “monoclonal” antibodies are refined into potential therapies that — with laser-like focus — can latch onto and “neutralize” specific viruses.
The VVC is perhaps best known for isolating in early 2020 — and in a record 25 days — monoclonal antibodies that neutralize the COVID-19 virus.
In late 2021, the Food and Drug Administration approved a long-acting combination of antibodies discovered at VUMC and developed by the global biopharmaceutical company AstraZeneca for emergency use to prevent COVID-19 in high-risk adults and children.
Five years earlier, VVC researchers reported the isolation of monoclonal antibodies that were cross-reactive for monkeypox (now called mpox), cowpox and the variola virus (smallpox).
Anitbodies against mpox
This summer the World Health Organization declared mpox, which is surging in parts of Africa, a public health emergency of international concern. Vaccines are in short supply. Crowe said his group is working with the federal government and industry to advance to clinical testing antibodies they had isolated in 2016.
“We already did the discovery work,” said Crowe, the Ann Scott Carell Professor and professor of Pediatrics and Pathology, Microbiology & Immunology. “They’re ready for clinical trials at the moment we need them, instead of starting from scratch.”
Earlier, VVC researchers isolated neutralizing antibodies against enterovirus D68, which has been linked to a rare but devastating polio-like illness in children, acute flaccid myelitis. After further development and testing in partnership with two biotechnology companies, VUMC launched the first-in-human trial of one of the antibodies in June 2024.
Hantavirus sprint
Also in June, the VVC began a “sprint” sponsored by the U.S. Department of Defense to discover neutralizing antibodies against hantaviruses, for which there currently are no approved vaccines or specific treatments. Transmitted primarily by rodents, hantaviruses can cause severe upper respiratory disease with a fatality rate approaching 40%.
Long before COVID-19, Crowe and his colleagues were advocating for a broad-based, proactive program of antibody and vaccine discovery and development. ReVAMPP, he said, is a big step in the right direction.






No comments yet