The symbiotic homeostasis of the intestinal microbiota, often referred to as the ‘forgotten organ’, plays an important role in host health.
Clinical data have shown that a diverse population of intestinal bacteria can enter the circulation through the mucosal system, accumulate and colonize in various types of tumor tissues, forming ’intratumoral microbiota’ that proliferates with tumor progression. The intestinal and intratumoral microbiota profoundly impacts tumor initiation, progression, and response to treatment through modulating oncogenic signaling pathways, promoting mutagenesis, altering the metabolism of chemotherapeutics, and shaping the host immune response.
Given the intricate links between microbiota and cancer, the development of antitumor therapies involving tumor-associated microbiota regulation is of crucial significance, especially for solid tumors such as colorectal cancer that are susceptible to microbial invasion.
Inulin-based hydrogel
In this study, published in Research, the authors developed an orally administered inulin-based hydrogel with colon-targeting and colon-retention effects, containing hollow MnO2 nanocarrier loaded with the chemotherapeutic drug oxaliplatin (Oxa@HMI).
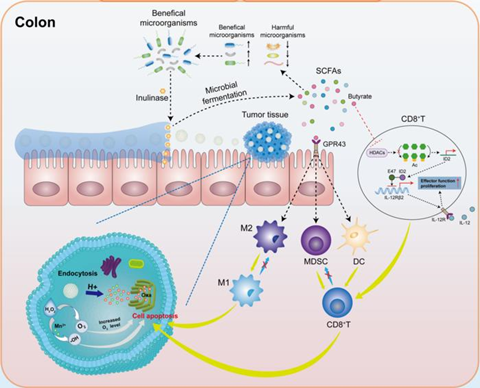
The inulin gelatinous matrix promoted the accumulation and concentration of the active ingredient at the lesion site through enhancing bioadhesion and prolonging colon retention. This facilitated the specific degradation of inulin in Oxa@HMI by inulinase secreted by beneficial bacteria in the colon, leading to the production of SCFAs. The SCFAs, in turn, initiated a cascade of anti-tumor immune responses by modulating the balance between harmful and beneficial bacteria.
On the other hand, degradation of the inulin gel matrix facilitated the colorectal cancer site-targeted exposure of internal nanomedicine Oxa@HM, which was degraded in the acidic TME to generate Mn2+ and O2, and release the loaded Oxa. The generated Mn2+ and O2 further exacerbated the oxidative stress levels in the TME through generating ROS to achieve significant tumor growth inhibition (Fig. 1).
Good structural stability
The authors first characterized the synthesized Oxa@HMI nanoparticles and verified their performance in vitro, respectively. The results showed that Oxa@HMI nanoparticles could maintain good structural stability in simulated gastrointestinal fluids, allowing the intact nanoparticles to be delivered to the colorectal tumor site. Subsequently, the protective layer of inulin on the surface would be degraded by inulinase secreted by beneficial bacteria in the TME, thereby fully exposing the internal nanodrugs Oxa@HM to exert its anti-tumor effects. After co-incubation with inulinase, the Oxa@HMI nanoparticles demonstrated excellent acid-responsive degradation performance and exhibited good ability to generate ROS and O2 at the solution level (Fig. 2A-H).
Subsequently, they evaluated the intracellular bio-functions of Oxa@HMI nanoparticles in tumor cells. The results showed that after co-incubation with inulinase, Oxa@HMI nanoparticles were able to efficiently generate O2 and ROS in tumor cells, which contributed to the enhanced therapeutic efficacy of Oxa. Additionally, Oxa@HMI nanoparticles (co-incubated with inulinase) exhibited significant targeted cytotoxicity against tumor cells and significantly promoted apoptosis in tumor cells (Fig. 2I-L).
Then, for the convenience of oral administration and gastrointestinal drug delivery, they constructed Oxa@HMI hydrogel by adjusting the modification ratio of inulin on Oxa@HMI nanoparticles to achieve better adhesion to the intestinal mucosal layer and prolong intestinal retention time (Fig. 3A-C). We further evaluated the in vivo biodistribution of Oxa@HMI hydrogel in orthotopic colorectal cancer mice. The results showed that compared to other groups, Oxa@HMI hydrogel, due to its adhesive properties, could adhere to the intestinal mucosa, thereby prolonging the retention time of nanodrugs in the colon, resulting in increased accumulation and concentration of nanodrugs in colon tumors (Fig. 3D-H).
In vivo effect
Immediately thereafter, the authors validated the in vivo anti-tumor effect of Oxa@HMI hydrogel in both orthotopic colorectal cancer mice and subcutaneous colorectal cancer mice. The results showed that Oxa@HMI hydrogel, with colon targeting and colon retention properties, demonstrated good anti-tumor efficacy in both mouse models by intervening in the microbial environment within the tumor tissue, improving the efficiency of chemotherapy and activating anti-tumor immune responses (Fig. 4).
Next, they assessed the variation of microbiota in the intestinal tract and tumor tissues of orthotopic colorectal cancer mice by high-throughput 16S rRNA gene sequencing, respectively. The results showed that in the intestinal tract and tumor tissues of orthotopic colorectal cancer mice, oral administration of Oxa@HMI hydrogel significantly increased the relative abundance of beneficial bacteria producing SCFAs, while significantly reducing the relative abundance of harmful bacteria promoting tumor progression (Fig. 5).
Finally, they analyzed the level of immune cell infiltration within the tumors of orthotopic colorectal cancer mice using flow cytometry. The results showed that the microbial metabolites of Oxa@HMI hydrogel, SCFAs, triggered a series of anti-tumor immune responses by binding to GPR43 in the intestine and activating the HDACs/ID2/IL-12 receptor pathway in CD8+ T cells: significantly increasing the infiltration levels of CD8+, IFN-γ+CD8+ and CD4+ T cells in tumor tissues, effectively inducing the maturation of DCs, significantly promoting the polarization of tumor-associated M2 macrophages into pro-inflammatory M1 macrophages, and significantly reducing the frequency of immunosuppressive MDSCs (Fig. 6).
Microbiota-targeted drug delivery
In this study, the authors have established, for the first time, a microbiota-targeted drug delivery system Oxa@HMI hydrogel that exhibited high efficiency in colorectal cancer targeting and colon retention.
Oxa@HMI hydrogel demonstrated excellent anti-tumor efficacy in both orthotopic colorectal cancer mice and subcutaneous colorectal cancer mice by intervening in the microbial environment within the tumor tissue, improving the efficiency of chemotherapy and activating anti-tumor immune responses, and exhibited remarkable biosafety, holding great potential for future clinical translation and treatment of colorectal cancer that is susceptible to microbial invasion.







No comments yet