Lignans are low molecular weight polyphenolic compounds with important antitumor and antiviral properties. However, their low amounts in medicinal plants and complex structures make sustainable production through plant extraction and chemical synthesis challenging, limiting their availability to meet market demand.
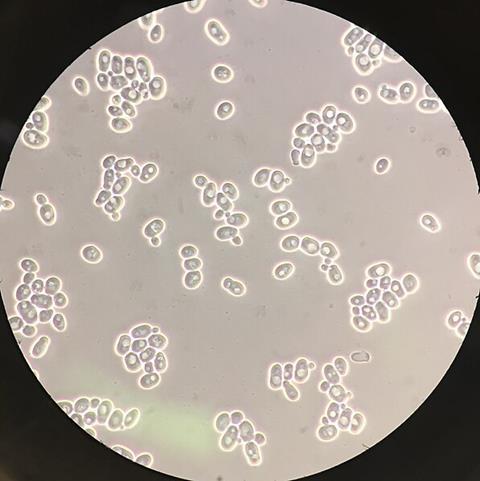
In a study published in Nature Chemical Biology, a research group led by Prof. Zhou Yongjin from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences, collaborating with Prof. Zhang Lei and Prof. Chen Wansheng from the Naval Medical University, has achieved the biosynthesis of the antiviral ingredient lignan glycoside in yeast Saccharomyces cerevisiae.
READ MORE: Engineered yeast strain can selectively overproduce carotenoids
READ MORE: Scientists synthesize precursors of powerful anti-cancer drug in yeast cells
Researchers constructed a synthetic yeast consortium inspired by plant metabolism. By mimicking the spatial and temporal regulation of plant biosynthesis, they designed a system with obligated mutualism, enabling metabolic division of labor among different yeast strains.
Reduced side reactions
This approach effectively reduced side reactions caused by the broad substrate spectrum of 4-coumarate: CoA ligase and improved the efficiency of metabolic flux toward the target product. Based on this innovative strategy, researchers addressed challenges related to side reactions and metabolic network promiscuity.
Besides, researchers designed two auxotrophic yeast strains (met15Δ and ade2Δ) to form a mutualistic relationship, cross-feeding metabolites while dividing the biosynthetic pathway into upstream and downstream. This enabled the de novo synthesis of lariciresinol diglucoside through over 40 enzymatic reactions.
“Our work demonstrates that Saccharomyces cerevisiae auxotrophic strains spontaneously establish a mutualistic community for the heterologous synthesis of complex active ingredients in traditional Chinese medicine. And this strategy is expected to be extended to the design of other stable cooperative yeast cell systems to accomplish complex bioengineering tasks,” said Prof. Zhou.





No comments yet