While H5N1 avian influenza virus taken from infected cow’s milk makes mice and ferrets sick when dripped into their noses, airborne transmission of the virus between ferrets — a common model for human transmission — appears to be limited.
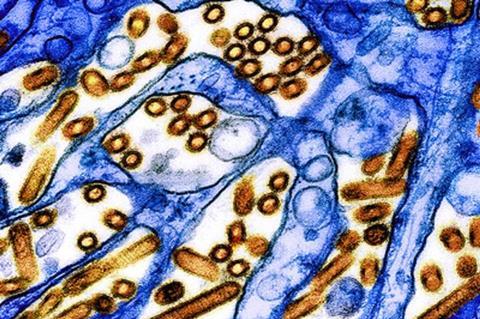
These and other new findings about the strain of H5N1 circulating among North American dairy cattle this year come from a set of laboratory experiments led by University of Wisconsin–Madison researchers, reported in the journal Nature. Together, they suggest that exposure to raw milk infected with the currently circulating virus poses a real risk of infecting humans, but that the virus may not spread very far or quickly to others.
“This relatively low risk is good news, since it means the virus is unlikely to easily infect others who aren’t exposed to raw infected milk,” says Yoshihiro Kawaoka, a UW–Madison professor of pathobiological sciences who led the study alongside Keith Poulsen, director of the Wisconsin Veterinary Diagnostic Laboratory, and with collaborators at Texas A&M University, Japan’s University of Shizuoka and elsewhere.
Mice and ferrets
Kawaoka cautioned, however, that the findings represent the behavior of the virus in mice and ferrets and may not account for the infection and evolution process in humans.
In their experiments, the UW–Madison team found that mice can become ill with influenza after drinking even relatively small quantities of raw milk taken from an infected cow in New Mexico.
Kawaoka and his colleagues also tested the bovine H5N1 virus’s ability to spread through the air by placing ferrets infected with the virus near but out of physical contact with uninfected ferrets. Ferrets are a common model for understanding how influenza viruses might spread among humans because the small mammals exhibit respiratory symptoms similar to humans who are sick with the flu, including congestion, sneezing and fever. Efficient airborne transmission would signal a serious escalation in the virus’s potential to spark a human pandemic.
Antibodies found
None of the four exposed ferrets became ill, and no virus was recovered from them throughout the course of the study. However upon further testing, the researchers found that one exposed ferret had produced antibodies to the H5N1 virus.
“That suggests that the exposed ferret was infected, indicating some level of airborne transmissibility but not a substantial level,” Kawaoka says.
Separately, the team mixed the bovine H5N1 virus with receptors — molecules the virus binds to in order to enter cells — that are typically recognized by avian or human influenza viruses. They found that bovine H5N1 bound to both types of molecules, representing one more line of evidence of its adaptability to human hosts.
Limited cases
While that adaptability has so far resulted in a limited number of human H5N1 cases, previous influenza viruses that caused human pandemics in 1957 and 1968 did so after developing the ability to bind to receptors bound by human influenza viruses.
Finally, the UW–Madison team found that the virus spread to the mammary glands and muscles of mice infected with H5N1 virus and that the virus spread from mothers to their pups, likely via infected milk. These findings underscore the potential risks of consuming unpasteurized milk and possibly undercooked beef derived from infected cattle if the virus spreads widely among beef cattle, according to Kawaoka.
“The H5N1 virus currently circulating in cattle has limited capacity to transmit in mammals,” he says. “But we need to monitor and contain this virus to prevent its evolution to one that transmits well in humans.”
Backdrop to research
In March 2024, an outbreak of HPAI H5N1 was reported among U.S. dairy cattle which spread across herds and led to fatal infections among some cats on affected farms, spillover into poultry, and four reported infections among dairy workers. The HPAI H5N1 viruses isolated from affected cattle are closely related to H5N1 viruses that have circulated in North American wild birds since late 2021. Over time, those avian viruses have undergone genetic changes and have spread throughout the continent causing outbreaks in wild birds and mammals—sometimes with mortality rates and suspected transmission within species.
To better understand the characteristics of the bovine H5N1 viruses, researchers from the University of Wisconsin-Madison, Japan’s Shizuoka and Tokyo Universities, and Texas A&M Veterinary Medical Diagnostic Laboratory conducted experiments to determine the ability of bovine HPAI H5N1 to replicate and cause disease in mice and ferrets, which are routinely used for influenza A virus studies. Ferrets are thought to be a good model for understanding potential influenza transmission patterns in people because they exhibit similar clinical symptoms, immune responses and develop respiratory tract infections like humans.
The researchers intranasally administered to mice doses of bovine HPAI H5N1 influenza of increasing strength (5 mice per dosage group), and then monitored the animals for body weight changes and survival for 15 days. All the mice that received the higher doses died of infection. Some of the mice that received lower doses survived, and those that received the lowest dose experienced no body weight loss and survived.
Vietnamese strain
The researchers also compared the effects of the bovine HPAI H5N1 virus to a Vietnamese H5N1 strain that is typical of H5N1 avian influenza virus in humans and to an H1N1 influenza virus, both delivered intranasally to mice. The mice that received either the bovine HPAI H5N1 virus or the Vietnamese avian H5N1 virus experienced high virus levels in respiratory and non-respiratory organs, including in the mammary glands and muscle tissues, and sporadic detection in the eyes. The H1N1 virus was found only in the respiratory tissues of the animals. Ferrets intranasally infected with the bovine HPAI H5N1 virus experienced elevated temperatures and loss of body weight. As with the mice, the scientists discovered high virus levels in the ferrets’ upper and lower respiratory tracts and other organs. Unlike the mice, however, no virus was found in the ferrets’ blood or muscle tissues.
“Together, our pathogenicity studies in mice and ferrets revealed that HPAI H5N1 derived from lactating dairy cattle may induce severe disease after oral ingestion or respiratory infection, and infection by either the oral or respiratory route can lead to systemic spread of virus to non-respiratory tissues including the eye, mammary gland, teat and/or muscle,” the authors write.
Respiratory droplets
To test whether bovine H5N1 viruses transmit among mammals via respiratory droplets, such as emitted by coughs and sneezes, the researchers infected groups of ferrets (four animals per group) with either bovine HPAI H5N1 virus or H1N1 influenza, which is known to transmit efficiently via respiratory droplets. One day later, uninfected ferrets were housed in cages next to the infected animals. Ferrets infected with either of the influenza viruses showed clinical signs of disease and high virus levels in nasal swabs collected over multiple days. However, only ferrets exposed to the H1N1-infected group showed signs of clinical disease, indicating that the cow influenza virus does not transmit efficiently via respiratory droplets in ferrets.
Typically, avian and human influenza A viruses do not attach to the same receptors on cell surfaces to initiate infection. The researchers found, however, that the bovine HPAI H5N1 viruses can bind to both, raising the possibility that the virus may have the ability to bind to cells in the upper respiratory tract of humans.
“Collectively, our study demonstrates that bovine H5N1 viruses may differ from previously circulating HPAI H5N1 viruses by possessing dual human/avian-type receptor-binding specificity with limited respiratory droplet transmission in ferrets,” the authors said.
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, funded the work of the University of Wisconsin-Madison researchers.




No comments yet