The Innovation Center of NanoMedicine and Prof. Nishiyama’s group at the Laboratory for Chemistry and Life Science, Institute of Science Tokyo have demonstrated, for the first time in the world using mice, the ability to overcome significant challenges in gene therapy using adeno-associated virus vectors (AAV), specifically production of neutralizing antibodies and hepatotoxicity, by employing a novel smart nanomachine equipped with AAV.

The research results were published online in the academic journal ACS Nano on February 4, 2025. This study was conducted as part of the activities of a national program “COI-NEXT” at Kawasaki (Project CHANGE), supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Science and Technology Agency (JST), with Project Leader, Prof. Takanori Ichiki, the University of Tokyo Graduate School of Engineering (also serving as a Research Director at iCONM).
The top authors of the paper are Assistant Professor Yuto Honda from the Laboratory for Chemistry and Life Science at Institute of Science Tokyo (also serving as a Senior Research Scientist at iCONM) and Shuhei Nagao (who was a master’s student at the time of the research), both of whom are noted for their equal contributions. Dr. Hiroaki Kino, a Principal Research Scientist at iCONM, and Prof. Nishiyama are listed as corresponding authors alongside Prof. Honda, while other researchers from iCONM are acknowledged as co-authors.
Tannic acid powered nanomachine
The research team designed a nanomachine equipped with adeno-associated virus vectors (AAV) by combining tannic acid, a component found in wine and tea, with a precision-synthesized polymer made from phenylboronic acid. Using this approach, they successfully overcame the challenges of “reduced gene transfer efficiency due to neutralizing antibodies” and “hepatotoxicity resulting from accumulation in the liver” in mice for the first time in the world.
READ MORE: Complement system response to AAV vector gene therapy
READ MORE: Novel nanosensing technique offers quality control of viral vectors in gene therapy
AAVs are clinically applied as gene therapy vectors, but it is known that patients with neutralizing antibodies against AAV do not achieve sufficient gene transfer efficiency, limiting the treatable patient population and the possibility of multiple administrations. The research team focused on the property of tannic acid, a natural component found in wine and other sources, to easily adhere to biomolecules. By combining it with a precision-synthesized polymer made from phenylboronic acid, they developed a novel AAV-equipped nanomachine.
Gene transfer activity
This AAV-equipped nanomachine demonstrated sufficient gene transfer activity even in the presence of AAV neutralizing antibodies and was also shown to suppress the increase of liver toxicity markers caused by AAV9 by inhibiting AAV accumulation in the liver. Furthermore, this nanomachine exhibited gene transfer efficiency to the central nervous system comparable to that of AAV alone, indicating its potential for significant gene therapy effects.
The findings from this research are expected to be applied as a new therapeutic approach to viral vector treatments, which are currently limited by neutralizing antibodies.
Gene therapy
Gene therapy is a treatment method that involves the administration of genes or gene-modified cells into the human body for the purpose of treating diseases. Among these, viral vectors are used as a method of gene delivery that utilizes the mechanism by which viruses infect cells. Adeno-associated virus vectors (AAV vectors), which are used in gene therapy, can introduce genes into various cell types and maintain long-term gene expression, making them clinically applicable for intractable diseases such as spinal muscular atrophy, Duchenne muscular dystrophy, and hemophilia.
However, the majority of adults naturally possess neutralizing antibodies against AAV, and once AAV is administered, it leads to the production of neutralizing antibodies, which restricts the number of patients who can receive treatment and limits the possibility of multiple administrations. Additionally, high doses of AAV administration have been shown to be toxic to the liver and kidneys, with reports of fatalities in clinical trials.
Addressing the challenges
To address these challenges, there have been attempts to modify the capsid of viral vectors with polyethylene glycol (PEG) or to encapsulate AAV within delivery carriers such as liposomes. However, these approaches can inhibit the intracellular uptake of the viral vectors, leading to decreased gene delivery efficiency.
Therefore, there is a need for an AAV delivery system that can evade neutralizing antibodies in the bloodstream, reduce accumulation in the liver, and control gene delivery to target cells.
Nanomachine background
To evade neutralizing antibodies from AAV, the research group developed an AAV-equipped nanomachine by combining tannic acid with precision-synthesized polymers. Tannic acid, a type of polyphenol abundantly found in wine and tea, interacts with biomolecules such as proteins and viral vectors through hydrophobic interactions and hydrogen bonding, forming complexes. Additionally, tannic acid is a natural ingredient that has garnered attention as a material for pharmaceuticals due to its excellent biodegradability and biocompatibility.
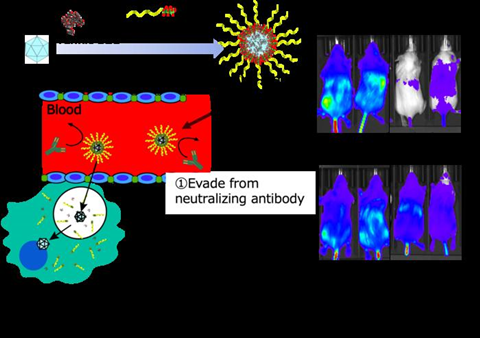
Focusing on these outstanding properties of tannic acid, Honda and colleagues have previously reported on a biomolecule delivery nanomachine that combines tannic acid with a precision-synthesized polymer containing phenylboronic acid that forms esters with tannic acid (Y Honda, et al. ACS Appl. Mater. Interfaces 2021, 13, 54850−54859). This nanomachine can be easily formed by simply mixing the desired molecules with tannic acid and the precision-synthesized polymer in water, and it can release the loaded molecules at the acidic pH found inside cells, demonstrating sufficient activity.
How it worked
The research group aimed to load AAV into this nanomachine to tackle the challenges in therapy (neutralizing antibodies, hepatotoxicity) without compromising the activity of the AAV. When AAV serotype 9 (AAV9) was systemically administered to mice with AAV neutralizing antibodies, the gene transfer efficiency in the brain and liver decreased to 5-15%. However, by loading AAV9 into the nanomachine, the gene transfer efficiency in the brain and liver upon systemic administration increased to approximately 50-60%, significantly reducing the loss of activity compared to AAV9 alone. Furthermore, this nanomachine successfully limited the impact on the liver to below 10%, effectively suppressing the hepatotoxicity associated with high-dose AAV9 administration.
In this way, the nanomachine not only overcomes the challenges of gene therapy viruses but also demonstrates gene transfer efficiency in the brain comparable to that of AAV9 alone, addressing the issues posed by conventional delivery carriers that reduce AAV activity, and thus substantial gene therapy effects are expected.
Additionally, by combining microbubbles with focused ultrasound irradiation, the research successfully enhanced the selectivity of gene transfer to the brain by sixfold.
New approach
- By simply mixing tannic acid, found in wine, with a precision-synthesized polymer in an aqueous solution, a nanomachine equipped with adeno-associated virus vectors (AAV) was created.
- The AAV-equipped nanomachine overcame the challenges of inactivation by neutralizing antibodies and hepatotoxicity due to accumulation in the liver, which are issues in viral vector gene therapy.
- This nanomachine demonstrated gene delivery functionality in the brain comparable to that of AAV alone.
- By combining this nanomachine with microbubbles and focused ultrasound irradiation, the efficiency of gene delivery to the brain using AAV was successfully increased sixfold.
Research potential
- This nanomachine can suppress the inactivation of gene therapy viruses due to neutralizing antibodies, which is a challenge in clinical applications, potentially increasing the number of patients eligible for AAV-mediated gene therapy.
- Additionally, since this nanomachine can maintain gene delivery to the brain while suppressing hepatotoxicity associated with high doses of AAV, it allows for an increase in the dosage, thereby enhancing the therapeutic effect.
- The AAV-equipped nanomachine can be easily formed by simply mixing AAV, tannic acid, and precision-synthesized polymers in water, making the preparation straightforward. Both tannic acid and the polymer exhibit excellent biocompatibility, which facilitates their application in clinical settings.
- Further structural optimization of the precision-synthesized polymer is expected to enhance the ability to evade neutralizing antibodies, potentially enabling multiple administrations of AAV9.
Topics
- adeno-associated virus vectors
- Asia & Oceania
- Disease Treatment & Prevention
- Early Career Research
- gene transfer
- Hiroaki Kino
- Immunology
- Innovation Center of NanoMedicine
- Innovation News
- Institute of Science Tokyo
- Microbial Biotechnology
- nanomachines
- neutralizing antibodies
- One Health
- Pharmaceutical Microbiology
- Shuhei Nagao
- Takanori Ichiki
- tannic acid
- University of Tokyo
- Viruses
- Young Innovators
- Yuto Honda




No comments yet