Researchers from Durham University, Jagiellonian University (Poland) and the John Innes Centre have achieved a breakthrough in understanding DNA gyrase, a vital bacterial enzyme and key antibiotic target.

This enzyme, present in bacteria but absent in humans, plays a crucial role in supercoiling DNA, a necessary process for bacterial survival.
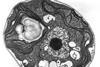
Using high-resolution cryo-electron microscopy the researchers reveal unprecedented detail of gyrase’s action on DNA, potentially opening doors for new antibiotic therapies against resistant bacteria.
The research is published in Proceedings of the National Academy of Sciences (PNAS).
Tiny molecular machine
DNA gyrase operates like a tiny molecular machine, carefully twisting and stabilising bacterial DNA. This twisting, known as supercoiling, is similar to winding an elastic band: as it twists, it coils tighter and tighter.
Unlike a band that would unwind if released, DNA gyrase stabilises DNA’s twisted form, making it functional for bacteria.
The enzyme wraps DNA in a ‘figure-of-eight’ loop, then precisely breaks and passes strands through each other, resealing them afterward. This is a delicate process—if the DNA remained broken, it would be lethal to the bacteria.
READ MORE: Dual action antibiotic could make bacterial resistance nearly impossible
READ MORE: Sugar cane pathogen delivers promising new antibiotic candidate
Antibiotics such as fluoroquinolones exploit this vulnerability by preventing the DNA resealing, which kills the bacterial cell. However, resistance to these antibiotics is growing, so a deeper understanding of how gyrase functions is urgently needed.
Snapshot at work
Using state-of-the-art cryo-electron microscopy, the team captured a snapshot of gyrase at work, revealing how it wraps DNA through outstretched protein arms to form the figure-of-eight shape.
This finding updates the conventional view of gyrase’s mechanism, which has been studied for decades. The images show the enzyme as a highly coordinated, multi-part system, with each piece moving in a precise sequence to achieve DNA supercoiling.
Reflecting on the study findings, co-author Professor Jonathan Heddle of Durham University said: “The results suggested the exact position and the order of the complex moving parts of the enzyme during when the supercoiling process occurs were not quite as we previously thought, and this could impact how we design new inhibitors.”
New antibiotics
This discovery not only advances our knowledge of bacterial biology but also holds promise for new antibiotics designed to block gyrase in a more targeted way, bypassing existing resistance mechanisms.
With this high-resolution structure as a guide, researchers aim to take additional snapshots of the enzyme in various stages, building a molecular movie of how gyrase works.
This detailed approach could aid in the development of next-generation antibiotics that are more precise and effective in stopping bacterial infections.
‘Structural basis of chiral wrap and T-segment capture by Escherichia coli DNA gyrase’, (2024), Elizabeth Michalczyk, Zuzanna Pakosz-Stępień, Jonathon D. Liston, Olivia Gittins, Marta Pabis, Jonathan G. Heddle, and Dmitry Ghilarov, PNAS.




No comments yet