The Cullin family proteins have been shown to act as internal components of the ubiquitin-proteasome system, mediating the signaling pathways for protein degradation and the maintenance of cellular homeostasis. These proteins act as scaffold proteins in the E3 ubiquitin ligase complex to facilitate ubiquitin transfer to specific substrate proteins, thereby labeling them primarily for proteasomal degradation.

This targeted degradation process is crucial for multiple cellular functions, including cell cycle progression, signal transduction, and inflammation. Their dysfunction is associated with multiple diseases, for instance cancer and neurodegenerative diseases, highlighting their importance in modulating organism health.
Recently, the research group of Prof. Shanming Ji from School of Life Sciences, Anhui Agricultural University, published an article titled ”Drosophila Cul3 contributes to Diap2-mediated innate immune signaling for antimicrobial defense” on hLife, focusing on the functional involvement and mechanism of Cullin-3 (Cul3) protein in antimicrobial immune defense.
Potential regulator
The research team initially identified Cul3 as a potential regulator in the Drosophila immune deficiency (IMD) signaling pathway. Subsequently, they used the UAS-Gal4 gene expression system and combined a series of genetics, immunology and molecular biology approaches to confirm that Cul3 plays an essential role in the Drosophila antimicrobial immune defense.
READ MORE: Immune systems develop ‘silver bullet’ defences against common bacteria
READ MORE: Researchers develop a new toolkit in fruit flies to study Zika virus
To investigate the molecular mechanism by which Cul3 mediates the host antimicrobial immune response, the research team used immunoprecipitation plus proteomic analysis to search the Cul3 interactome and identified Death-associated inhibitor of apoptosis 2 (Diap2) as a potential Cul3-interacting protein. They further performed a combination of biochemical and molecular experiments and revealed that Cul3 enhances the E3 ubiquitin ligase activity of Diap2 and regulates the ubiquitination modification of Death related ced-3/Nedd2-like caspase (Dredd).
Functional role
In addition, the research team carried out a series of genetic and biochemical approaches and elucidated the functional role of the Cul3/Diap2/Dredd axis in regulating the Drosophila antimicrobial immune defense.
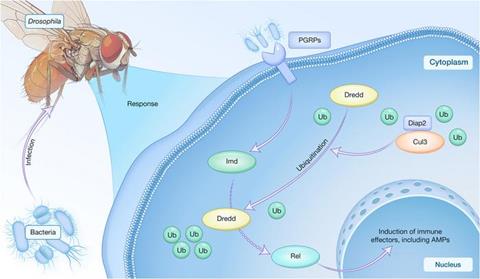
Finally, they proposed a Cul3/Diap2/Dredd-involved working model in modulating the Drosophila antimicrobial immune defense (Figure).
In conclusion, the research team have systematically explored the function and molecular mechanism of the Cullin family protein Cul3 in the Drosophila antibacterial immune defense. Given the high conservation of Cullin family proteins and innate immune signaling pathways in Drosophila and mammals, the findings will provide new ideas for the treatment of relevant diseases in clinic.





No comments yet