In a study published in Cell Reports Physical Science on Nov. 25, a research team led by Prof. Cai Lintao from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences reported its development of an innovative intelligent light-guided biohybrid system, the CTPA/siCSF1R system, to target tumor-associated macrophages (TAMs), thus enabling precise spatiotemporal siRNA delivery. This system enhances the tumor microenvironment and supports precise photoimmunotherapy.
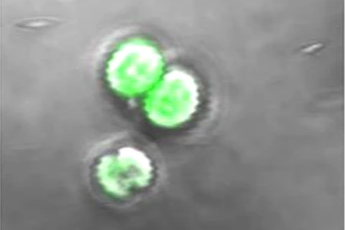
TAMs are crucial immune cells in the tumor microenvironment and key targets for tumor immunotherapy, with gene therapy being an important approach. However, existing gene carriers are unsatisfactory due to poor targeting, limited lysosomal escape, and the negative effects of a hypoxic immunosuppressive microenvironment, which limit their clinical efficacy.
In this study, researchers employed a living biological carrier, the cyanobacterium Synechocystis sp. PCC6803 (“cyan”), which exhibits self-driven tumor targeting and phototactic capabilities. These characteristics make it an excellent vehicle for precise drug delivery under controllable external forces. Consequently, cyan’s phototactic behavior, along with its ability to produce oxygen through photosynthesis, has potential for delivering nucleic acid drugs to solid tumors.
Biological carrier
The CTPA/siCSF1R system consists of a triblock polyamino acid (TPA) gene vector encapsulating siRNA, which is conjugated to the surface of photosynthetic cyanobacteria. It utilizes the inherent self-driven and phototactic abilities of cyanobacteria to achieve precise targeting of TAMs within the tumor microenvironment.
The light-guided production of reactive oxygen species by cyanobacteria and the protonation of TPAs disrupt the lysosomal membrane, facilitating the release of siRNA into the cytoplasm of TAMs. Meanwhile, oxygen produced by cyanobacteria through photosynthesis enhances the tumor microenvironment, promoting the efficacy of siRNA delivery and the reprogramming of TAMs.
READ MORE: Cyanobacteria can ‘grow’ stronger sand-based construction materials
READ MORE: Computer-aided biology can be deployed to develop tailored microbe communities
Experimental results show that the CTPA/siCSF1R system effectively reprograms TAMs to the M1 phenotype, promotes the production of pro-inflammatory cytokines, and induces a strong immune response that inhibits tumor growth. Furthermore, the system exhibits excellent biosafety and does not cause significant toxicity to the organism.
The system proposed in this study offers a promising approach for developing both efficient and safe nucleic acid delivery vectors as well as advancing tumor photoimmunotherapy.





No comments yet