Moffitt Cancer Center has launched a pioneering clinical trial for patients with late-stage non-small cell lung cancer whose disease has progressed following initial frontline standard of care immunotherapy that includes an immune checkpoint inhibitor alone or combined with chemotherapy. The innovative trial uses a novel oncolytic virus, MEM-288, in combination with the immune checkpoint inhibitor nivolumab.
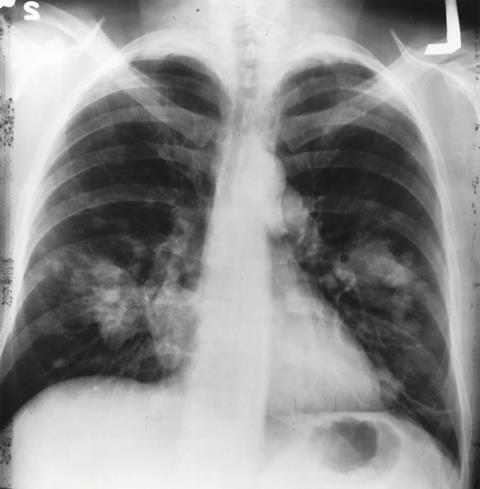
Spearheaded by Moffitt, in collaboration with Duke Cancer Institute, the trial follows the successful completion of a first-in-human phase 1a trial of MEM-288 for non-small cell lung cancer. The phase 1a trial demonstrated that MEM-288 safely shrank tumors and enhanced antitumor immunity in patients.
MEM-288, developed in partnership with Memgen Inc., is engineered from an adenovirus that not only targets and destroys tumor cells but also bolsters the immune system’s response against cancer by expressing two key proteins: IFNβ and a membrane-stable version of CD40 ligand (MEM40). Amer A. Beg, Ph.D., a research scientist in the Department of Immunology at Moffitt, has shown in both preclinical and clinical studies MEM-288’s significant potential in activating robust antitumor immunity.
Promising results
The recent phase 1a trial demonstrated promising results, with MEM-288 increasing both the density and efficacy of tumor-killing T cells. Importantly, it also enhanced systemic antitumor T cells capable of targeting metastatic sites beyond the injected tumors. Given the known limitations posed by immune checkpoints like PD-1, the combination with nivolumab is anticipated to further unlock MEM-288’s therapeutic potential through the synergistic activity of this combination.
“Combining MEM-288 with nivolumab represents a highly promising and innovative therapy in our fight against lung cancer. We are hopeful that this trial will lead to more effective and enduring treatment options for patients who currently have few,” said Andreas Saltos, M.D., medical oncologist in the Department of Thoracic Oncology at Moffitt who is leading the clinical trial.
Five-year grant
This trial’s significance is underscored by a recent R01 grant (R01CA283730) awarded to Beg by the National Cancer Institute. The $2.3 million five-year grant, “Defining impact of in situ activation of CD40 and type 1 interferon signaling on the TME and systemic T cell immunity in murine models and cancer patients,” will support further research into MEM-288. This includes investigating its systemic effects and identifying potential biomarkers for treatment efficacy and resistance.




No comments yet