Immune cells that eat bacteria in the body don’t stash them in specialized compartments as once thought, but turn them into critical nutrients that build proteins, create energy and keep the cells alive, according to a new study from scientists at the University of Colorado Anschutz Medical Campus.
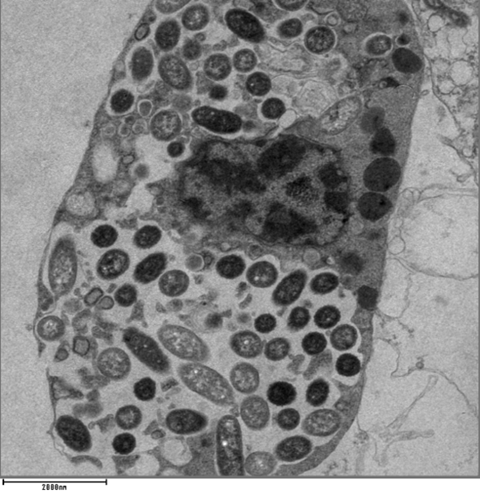
“We are what we eat,” said the study’s co-senior author Angelo D’Alessandro, Ph.D., professor of biochemistry and molecular genetics at the University of Colorado School of Medicine at CU Anschutz. “What we eat changes the composition of us and when immune cells eat bacteria the same thing happens to them.”
The study was published today in the journal Nature.
Inflammation response
The researchers also discovered that when these cells, called macrophages, eat live bacteria it triggers an inflammation response. But when they eat dead bacteria it doesn’t.
READ MORE: Researchers uncover new reasons to target neutrophils for tuberculosis therapy
READ MORE: Scientists deploy synthetic amyloids to figure out ways of targeting biofilms
“When phagocytic cells eat dead bacteria some of the small molecules they recycle tell them not to induce inflammation, that everything is going to be fine,” D’Alessandro said. “But when they eat live bacteria that signal is not there and it can induce inflammation which drives many diseases.”
The scientists delved deeply into the workings of these immune cells to better understand the `switches’ that turn inflammation on and off. They discovered a key protein complex called mTORC1 regulated how macrophages used the nutrients from the bacteria they consumed. They also found that dead bacteria contain a molecule known as cAMP that might tell immune cells that bacteria are dead so they can adjust their metabolism and better control inflammation.
Nature’s brakes
Chronic inflammation can drive everything from cancer and long COVID to chronic fatigue syndrome and shingles. These findings can help scientists and physicians develop therapies to better control this response.
“Over the next 10 years we will be dealing with more antibiotic resistant strains of bacteria,” D’Alessandro said. “Understanding that there are brakes that nature has evolved will help us eliminate or boost this response depending on the circumstances.”
The co-authors include Assistant Research Professor Julie A. Reisz Haines, Ph.D., of CU Anschutz, Parnika Mukherjee, Ph.D., of Charité–Universitätsmedizin Berlin, Germany as well as Juliette Lesbats, Ph.D., and Johan Garaude, Ph.D., of the University of Bordeaux in France.





No comments yet