Invasive pulmonary aspergillosis is a life-threatening fungal infection that occurs when conidia (spores) from the Aspergillus fungus are inhaled and establish an infection in the lungs, particularly in individuals with a weakened immune system, allowing the fungus to spread beyond the lung tissue into the bloodstream. Treatment options are scant. When the specific pathogen is Aspergillus fumigatus, mortality can be as high as 90%.
Researchers at the University of São Paulo (USP) in Brazil compared the full set of proteins present on the surface of A. fumigatus conidia and other closely related species that do not necessarily cause infection. They identified 62 proteins found solely in A. fumigatus.
An article on the study is published in the journal Nature Microbiology. The research project was led by scientists at the Ribeirão Preto School of Pharmaceutical Sciences (FCFRP-USP).
Protein can inhibit the production of immune cells
One of the proteins identified was glycosylasparaginase, which drew the researchers’ attention owing to its ability to inhibit the production of immune cells. When mutants of the fungus that did not produce glycosylasparaginase came into contact with mouse cells, they triggered an increase in the secretion of proinflammatory cytokines – proteins released by immune cells to alert the host to invading microorganisms.
READ MORE: Researchers discover key gene regulating virulence of fungus that causes severe lung infections
READ MORE: Aspergillus fumigatus adapts to life in humans - and shapes their lung microbiome
In contrast, murine cells infected by the wild-type fungus, in which all genes functioned normally, did not secrete high levels of these cytokines, suggesting that glycosylasparaginase plays a major role in modulating and reducing cytokine production so that the fungus has time to initiate and consolidate the infection.
“This is the first study to characterize the role of glycosylasparaginase in fungi. In humans, the gene mutation that produces this enzyme causes a rare neurodegenerative disease (aspartylglucosaminuria), in which glycoasparagine accumulates in various tissues of the body, including the central nervous system. This causes developmental delay, psychomotor impairment, intellectual disability, and finally premature death. Unfortunately, there is no treatment for this disease,” said Camila Figueiredo Pinzan, first author of the article and a researcher at FCFRP-USP.
When the researchers infected mice separately with the wild-type fungus and the mutant version (which did not produce glycosylasparaginase), they found that the fungal burden in the lungs of the group infected with the mutant version was lower. “This could mean that lack of the enzyme glycosylasparaginase makes the fungus more prone to elimination by the immune system,” Pinzan said.
Potential targets for future interventions
The study was part of a project supported by FAPESP and led by Gustavo Henrique Goldman, a professor at FCFRP-USP.
“Studies like this are fundamental to understand how fungi cause infection and identify novel potential targets for drug development. Conidia are the fungal cells that make first contact with the human respiratory system and initiate infection. These findings could represent a first step toward acquisition of the capability to combat the invasion in the initial stages,” Goldman said.
Glycosylasparaginase is only one of the 62 proteins identified in the study, he stressed. The others are being analyzed by his lab and exhibit varying degrees of promise as potential targets for future interventions.
Non-pathogenic fungi
To find out which of these proteins were present in A. fumigatus but not in other species of Aspergillus, the researchers analyzed conidia of three other species using proteomics: A. fischeri and A. oerlinghausenensis, which do not cause disease in humans, and A. lentulus, which can cause disease but is much less virulent than A. fumigatus.
“Although the similarity between these species can reach 95%, A. fumigatus can kill up to 90% of infected individuals, whereas for the others there are no reports of human infection, except for A. lentulus, which rarely causes disease,” said Thaila Reis, penultimate author of the article and a researcher at FCFRP-USP.
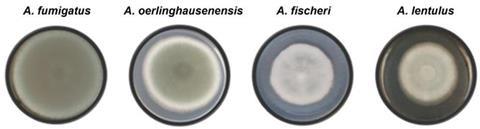
Understanding how fungi cause infection and become more virulent is essential to combat known pathogens and others that may emerge. With this in mind, the research group analyzed A. fischeri, a species not usually considered pathogenic. The findings of this study are published in Communications Biology.
Mechanisms of virulence in ’non-pathogens’
They assessed the pathogenic potential of 16 strains of A. fischeri, one of the four species analyzed previously, in experiments involving cell and animal models, concluding that some strains are in fact capable of causing infection.
For David Rinker, first author of this study and a professor at Vanderbilt University in the United States, “fungal pathogenicity is not considered obligate, but rather opportunistic. Therefore, the potential for new, opportunistic fungal pathogens to make the ‘leap’ into the clinic may be greater than we previously realized.”
Looking for shared mechanisms of virulence in “non-pathogens” may shed light on how pathogens originate or even predict the emergence of novel pathogens, Rinker is quoted as saying in a post to VU’s website.
“Both studies point to the need for a broader perspective on fungal virulence that includes species hitherto considered non-pathogenic. They may harbor a hidden potential to cause disease that emerges under certain environmental conditions or in immunosuppressed people,” Goldman said.
Topics
- A. fischeri
- A. lentulus
- A. oerlinghausenensis
- aspartylglucosaminuria
- Aspergillus fumigatus
- Camila Figueiredo Pinzan
- conidia
- David Rinker
- FCFRP-USP
- Fungi
- glycosylasparaginase
- Gustavo Henrique Goldman
- Infectious Disease
- Invasive pulmonary aspergillosis
- One Health
- Research News
- Ribeirão Preto School of Pharmaceutical Sciences
- Thaila Reis
- The Americas
- University of São Paulo
- Vanderbilt University




No comments yet