Humans are protected by two branches of the immune system. Innate immunity provides built-in defense against widespread characteristics of bacteria and viruses, while adaptive immunity memorizes individual pathogens that a person has already encountered.

Vaccines teach the adaptive immune system about new pathogens without having to go through an actual infection. This has greatly contributed to human health, but requires a specific vaccine for each major pathogen.
Some vaccines not only teach the adaptive immune system about a specific pathogen, but also increase the overall vigilance of our body’s innate immune cells. The BCG vaccine, which teaches our adaptive immune system to fight tuberculosis bacteria, has been shown to reduce infant mortality independent of its protection against tuberculosis. This observation can be explained by the concept of “trained immunity” – the idea that innate immune cells can switch between dormant and vigilant states, and are more effective at fighting infection when in their vigilant state.
Not every immune cell needs training
Inducing trained immunity by drugs or vaccines could confer protection in times of high infection risk, for example following a major surgery or during future pandemics before tailored vaccines become available. However, trained immunity is highly variable between individuals, and it is not well understood who may profit from inducing trained immunity.
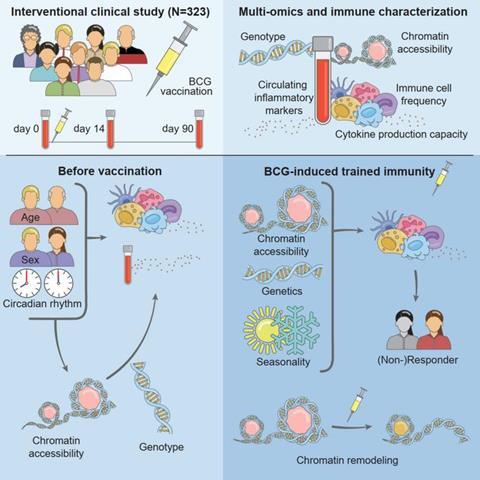
To investigate this issue, a team led by Mihai Netea (Radboud University Medical Center) and Christoph Bock (CeMM & Medical University of Vienna) vaccinated 323 healthy volunteers with BCG and analyzed the effects on the immune system. They found that the induction of trained immunity was most effective in individuals with dormant innate immunity, which was reflected in a characteristic epigenetic cell state that predicted the vaccine response (Moorlag SJCFM, Folkman L, ter Horst R, Krausgruber T, et al. Immunity. 2024).
The team identified 213 individuals as trained immunity responders and 78 as non-responders, based on whether or not their production of inflammatory mediators had increased at day 90 after BCG vaccination – at a point when the acute response has subsided, but trained immunity is expected to persist. Trained immunity responders produced fewer mediators before vaccination and started with more dormant innate immune cells than non-responders. In other words, the non-responders already had the higher immune vigilance that the BCG vaccine induced in the responders.
Epigenetic regulation
Both genetic and environmental factors contributed to this variability, but the most interesting differences were observed in the epigenetic states of the immune cells. Epigenetic cell states, implemented through changes in chromatin accessibility that make genes easier or harder to activate, reflect the regulatory plasticity of a cell and its ability to respond rapidly to changes in its environment, making them strong candidates for regulating trained immunity.
Indeed, in response to BCG vaccination, trained immunity responders gained open chromatin at genes involved in innate immunity, while non-responders carried such open chromatin independent of BCG vaccination, with no further increase following the vaccination. This finding explains how epigenetics allows immune cells to switch between different levels of immune vigilance, which contributes to the need to balance immune activity to provide protection against pathogens while avoiding unnecessary and harmful immune responses.
The study also clarifies a previously observed association between scar development at the site of BCG vaccination on the skin and lower child mortality. Previously, scar formation at the vaccine injection site was interpreted as a sign of a strong immune response to the vaccine. However, the team’s analyses offer an alternative explanation: it seems that scar formation reflects strong immunity prior to vaccination, and these individuals may be better protected against infections independent of BCG vaccination.
Enhancing immune vigilance
These results not only provide new insights into immune biology and the role of epigenetics, but also guide the development of future therapeutics. “We can envision a new class of drugs that deliberately wake up a dormant immune system,” Netea says. “Elderly people could receive a boost of their immune system prior to a planned hospital stay, and it may be possible to reactivate the suppressed immune system in patients with cancer. Several pharmaceutical companies are already pursuing ways to induce trained immunity without having to rely on the BCG vaccine.”
The new study provides important guidance for such endeavors. First, a better understanding of the biological pathways underlying trained immunity may uncover novel therapeutic targets. Second, the study shows that such therapeutics are only likely to benefit individuals with dormant innate immunity, who can be identified through chromatin profiling or functional immune assays. Third, no overshooting immune responses were observed in individuals with high innate immunity prior to vaccination, which bodes well for the safety of future trained immunity inducing drugs.
Bock summarizes: “Our study highlights the close connection between epigenetic cell states and trained immunity, allowing the human body to switch between vigilant and dormant innate immunity. This process is variable across individuals and may be exploited with precision medicine.”
The study “Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity” was published in Immunity.



No comments yet