Scientists have discovered that aberrant cellular metabolism in Candida fungi is linked to drug resistance, potentially opening up new possible pathways to antimetabolite therapies.
The study by researchers at the University of Kashmir is reported in the paper ‘Glucose metabolic reprogramming and modulation in glycerol biosynthesis regulates drug resistance in clinical isolates of Candida’, which has recently been accepted by the Journal of Applied Microbiology, an Applied Microbiology International publication.
It found that, similar to some human malignant tumours, there is a strong correlation between drug resistance and aberrant cellular metabolism in the opportunistic fungi belonging to the genus Candida.
This aberrant cellular metabolism confers antifungal resistance to these opportunistic fungi, pointing to the need to harness antimetabolite therapies alongside conventional antifungal drugs against these novel targets.
Grave threat
Lead author Dr Abdul Haseeb Shah said: “Fungal infections, particularly those caused by Candida, are evolving as grave threats to the healthcare system.
“With the mounting incidence of these opportunistic fungal infections, the limited availability of antifungal drug classes and the decreased response of certain fungi to these antifungals, the emergence of multiple drug resistance by these pathogens has posed a serious public health concern.
“In this context, the present study aimed to unravel the less explored mechanisms that govern this drug resistance at the molecular level in this group of pathogenic fungi.”
The team used proteomic analysis and subsequent studies to provide insights into the aberrant cellular metabolism that confers antifungal resistance.
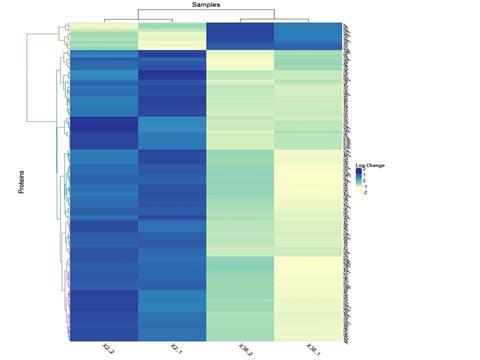
“Different Candida species display varying susceptibilities towards commonly used antifungals, and the development of antifungal resistance is a challenge for disease management, necessitating a better understanding of drug resistance,” Dr Shah said.
Proteomic analysis
“Proteomic analysis of C. albicans clinical isolate revealed the hexokinase 2 (Hxk2), the first rate-limiting glycolytic enzyme-dependent acquisition of drug resistance, wherein the elevated expression of Hxk2 was a key factor governing drug resistance via increased glucose uptake, ATP generation, and enhanced lactate production (somewhat similar to the Warburg effect, seen in human cells).
“We evaluated the Candida Hxk2 inhibitory effect of 3-Bromopyruvate (3-BP), a pharmacological inhibitor of Hxk2 in human cells. Interestingly, 3-BP treatment rescued glucose uptake, ATP production, and lactate production in these Candida isolates.
“Drug-resistant C. albicans also revealed a better G6pd mediated ROS scavenging as the resistant isolate showed a significant upregulation of G6pd that was directly associated with increased production of NADPH, and hence better ROS scavenging.
“In contrast, there was no such difference in NADPH production and ROS scavenging in C. glabrata isolates.
“We report G6pd as a strong possible therapeutic target in otherwise drug-resistant fungi. For the first time, we used a selective pharmacological inhibitor of G6pd (polydatin) and showed that it inhibited the PPP metabolic pathway and related pathogenicity in this group of pathogenic fungi.
“Polydatin treatment was able to inhibit G6pd expression and rescue ROS scavenging via NADPH depletion. Importantly, inhibiting Hxk2 and G6pd, also rescued FLC resistance in drug-resistant isolates of C. albicans.”
Drug resistance
In contrast, the C. glabrata-resistant isolate did not show any difference in the expression of Hxk2 and G6pd. Instead, a glycerol biosynthesis-mediated drug resistance was implicated in C. glabrata, imparting better adaptability to the pathogen in response to osmotic stress. “Mechanistically, such glycerol accumulation was driven via the HOG1-MAPK axis, wherein the phosphorylation of Hog1 (and hence its activation) led to the increased accumulation of intracellular glycerol,” Dr Shah said.
“Phosphorylated Hog1, in turn, controls the accumulation of glycerol by stimulating the transcription of glycerol-producing enzymes (Gpd1, Gpd2) and glycerol-proton symporter (Slt1), rapid closure of glycerol facilitator (Fps1) besides activating 6-phosphofructo-2-kinase (Pfk26/27) which was also confirmed by proteomic analysis.”
The rapid rise and geographic spread of Candida infections and multidrug resistance is of deep concern and emphasises the need for continued surveillance, rapid diagnostic testing, expanded laboratory testing capacity, deeper insights into drug resistance and hunt for a diversity of antimicrobial targets and therapies, Dr Shah said.
“On these objectives, this study has explored the metabolic regulation of drug resistance in C. albicans and C. glabrata, which revealed some deeper insights about the drug resistance mechanisms as well as the similarities and differences in the drug resistance mechanisms between these two fungal species,” he said.
“Further evaluation and study of these metabolic pathways and mechanisms will be very important steps to identify novel targets which can be utilised to curb the menace of antifungal drug resistance.”
Metabolic regulation
The study opens up a route for studying the basic metabolic regulation and its role in drug resistance in human pathogenic fungi, particularly the genus Candida.
“Although the study is novel in exploring and unravelling the role of some intermediary metabolic pathways in the acquisition of drug resistance in Candida, definitely further evaluation is warranted in this direction for the identification of more efficient alternate drug targets which can be put to trials,” Dr Shah said.
“Independent studies with a larger sample size, along with the use of robust modern techniques like CRISPR-Cas9 gene editing, Chip-seq, and in vivo approaches using selected gene modifications, will provide more validation for our results, which could become game changing in curbing the ever-increasing menace of drug-resistant fungal infections.”
The study, led by Dr. Peer Abdul Haseeb Shah, Assistant Professor at Department of the Bioresources at the University of Kashmir, J&K-India, was carried out with other co-authors, including Dr. Sajad Ahmad Padder, the first author of the study and collaborators from other institutions.
The study was mainly supported by the University of Kashmir and the financial support was provided by funding from extramural research grants awarded to Dr. Peer Abdul Haseeb Shah by the Department of Science and Technology, GoI under INSPIRE Faculty Award and by the Science and Engineering Research Board, GoI under the Early Career Research award.
‘Glucose metabolic reprogramming and modulation in glycerol biosynthesis regulates drug resistance in clinical isolates of Candida’ appears in the Journal of Applied Microbiology.







No comments yet