From safeguarding our food supply to preventing hospital infections, the battle against antibiotic-resistant bacteria is a growing challenge. Some bacteria can form biofilms, thick aggregates of millions of individual cells surrounded by protective mucus-like substances that easily adhere to surfaces. Forming such biofilms is a critical bacterial strategy to resist treatment.

The dense, layered colonies shield bacteria from immune cells and reduce the effectiveness of antiseptics and antibiotics. “Once a biofilm forms, its structure acts as a barrier, making it extremely difficult for drugs to penetrate and kill the bacteria,” explains Dr. Chisato Takahashi, a principal investigator at the National Institute of Advanced Industrial Science and Technology (AIST). The biofilms’ extraordinary resilience has motivated researchers to seek innovative solutions beyond traditional antibiotics.
READ MORE: Silver nanoparticles guarantee antimicrobial safe-tea
READ MORE: Nanoparticles with antibacterial action shorten duration of tuberculosis treatment
In their recently published paper in the journal Nanoscale, a team of scientists from the Okinawa Institute of Science and Technology (OIST) and AIST have developed a novel approach to fight against treatment-resistant bacteria.
Multiple mechanisms
To overcome the shortcomings of conventional antibiotics, the researchers developed a unique nanoparticle that combines multiple mechanisms to kill the bacteria. “We encapsulated our silver particles inside a polymer shell of Soluplus® and infused it with azithromycin, an antibiotic. This innovative encapsulation strategy makes the nanoparticle stable and highly effective in their antimicrobial activity,” says Dr. Takahashi.
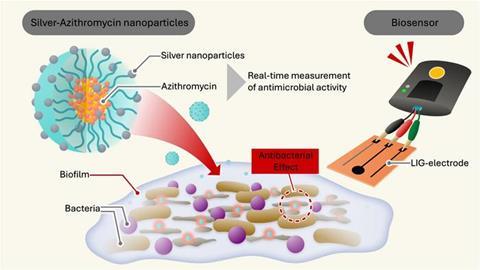
After demonstrating the stability of these nanoparticles in a previous study, it was time to test their effectiveness. “We chose two well-known bacteria that tend to cause problematic hospital-acquired infections: Escherichia coli and Staphylococcus epidermidis,” says Dr. Murali Mohan Jaligam, a postdoctoral scholar at OIST’s Micro/Bio/Nanofluidics Unit and the study’s first author.
These bacteria are notorious for forming resilient biofilms on surfaces like catheters and surgical implants, leading to severe, treatment-resistant infections inside the human body. Antibiotics act highly specifically in removing bacteria, which limits available treatment options during bacterial infection, a limitation that becomes especially critical when antibiotic resistance emerges.
Dual-action attack
In those situations, the cutting-edge nanoparticles can surpass conventional methods. “Our nanoparticles can deliver a dual-action attack—targeting bacterial cells with both antibiotic and silver ions. The encapsulating polymer ensures stability and prevents the nanoparticles from clumping, enhancing their effectiveness,” says Prof. Amy Shen, head of the OIST Micro/Bio/Nanofluidics Unit.
Only by combining silver, antibiotics, and polymer did the researchers give their nanoparticles these unique capabilities to penetrate and disrupt bacterial biofilms, “it’s not just any nanoparticle that can do the trick,” adds Dr. Takahashi.
The researchers used scanning electron microscopy and optical density measurements to observe how the nanoparticles disrupt biofilms. While these methods are well established, they can be time-consuming and require the sample to be stained with special dyes. Developing laser-induced graphene (LIG) electrodes allowed the team to overcome existing technical limitations.
Faster and more accurate
“We created a miniaturized, highly sensitive LIG electrode system capable of real-time monitoring bacterial activity,” explains Dr. Jaligam. These electrodes have large surface areas, giving bacteria an ideal base to form biofilms, and are highly conductive so that they can easily measure the flow of electrical charges. Because decaying bacteria create a different electrochemical signal than intact bacteria, the electrode can detect the bacteria cell’s breakdown as the electrical current changes. This method is faster and more accurate than traditional methods to assess antimicrobial activity and works without staining the bacteria.
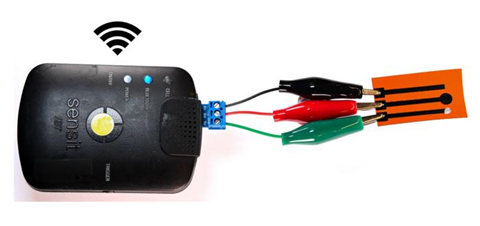
“Our LIG sensor technology offers an efficient, scalable, and cost-effective solution for detecting and managing bacterial contamination and biofilms,” notes Prof. Shen. These qualities open several fields for electrode use, such as cancer screening. Equally, nanoparticles have potential uses beyond combating hospital-acquired infections, for example, in coating medical devices to prevent biofilms from forming in the first place.
“Antibiotic resistance continues to pose a critical threat to global health, but breakthroughs like this offer a promising path forward. Our study shows the potential of collaborative, interdisciplinary research to address some of the most urgent and complex challenges we currently face in modern medicine,” says Prof. Shen.
Topics
- Amy Shen
- Antimicrobial Resistance
- Asia & Oceania
- Bacteria
- Biofilms
- Chisato Takahashi
- Economic Equality
- Infection Prevention & Control
- Innovation News
- Microbiological Methods
- Murali Mohan Jaligam
- National Institute of Advanced Industrial Science and Technology
- Okinawa Institute of Science and Technology
- One Health
- Rapid Diagnostics







No comments yet