Researchers from the Complexity Science Hub and the University of Veterinary Medicine Vienna have dissected the complex interactions involved in zoonoses, which affect worldwide over two billion people annually. They introduce the concept of a “zoonotic web,” a detailed network representation of the relationships between zoonotic agents, their hosts, vectors, food sources, and the environment.
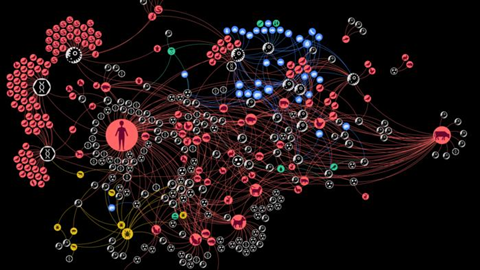
“Zoonotic diseases, which can be transmitted between animals and humans, are a significant public health concern, and our study highlights the importance of a holistic approach to understanding and managing these risks,” says epidemiologist and CSH researcher Amélie Desvars-Larrive.
Transmission context
The transmission of zoonoses to humans can occur through direct contact with saliva, blood, urine, or even feces of infected animals. For instance, a bite (for rabies), a scratch (for cat scratch disease), or skin contact (for skin fungi). Indirect transmission can also occur through bites from arthropod vectors – as with West Nile virus and tick-borne encephalitis – or through contact with contaminated objects, environments, or surfaces.
“For example, the food and water we consume can be potential routes of infection for zoonoses,” adds Desvars-Larrive, also an associate professor at the University of Veterinary Medicine, Vienna (Vetmeduni).
More than host-pathogen interaction
“Zoonotic diseases are often discussed in terms of host-pathogen interactions. Understanding the complex animal-human-environment interface remains a significant challenge,” explains the epidemiologist.
“As co-author Anja Joachim pointed out, simply studying the presence of a parasite in cat faeces, like Toxoplasma, doesn’t tell the whole story. Are we looking at the cat-environment, environment-human, or cat-human interface?. The concept of ‘interface’ remained unclear. This motivated us to develop a novel approach to zoonoses and demonstrate it through a case study,” adds Desvars-Larrive.
“We wanted to develop a method that investigates the interfaces where the exchange of circulating zoonotic pathogens takes place. This goes beyond host-pathogen interactions to consider other sources of infection, such as a contaminated environment, for example a sandpit, or contaminated food, often neglected when modeling zoonotic disease dynamics.”
Austrian data spanning almost 50 years
The researchers first carried out a systematic literature search on all documented interactions between zoonotic sources and pathogens in Austria between 1975 and 2022. From this, they created the “zoonotic web.” The results of the analysis were finally prepared in a dashboard by CSH data visualization expert Liuhuaying Yang.
The team identified six distinct communities of zoonotic agent sharing in Austria, influenced by highly connected infectious agents, proximity to humans, and human activities. The community including humans, the oldest domesticated species – including dogs, cats, sheeps, cattle, and pigs – and species that have adapted to live together with humans – for instance, house mouse – shares the most zoonotic agents, according to the study.
The findings also highlight the important role some animals, such as the wild boar, dog, domestic cat, yellow-necked field mouse, or the raccoon dog, and arthropods, especially ticks, play in “bridging” host communities. “Knowing which actors in the network are more influential than others can be very helpful in zoonotic disease surveillance programs, for example, as they could serve as sentinels for monitoring zoonotic agent circulation,” says Desvars-Larrive.
A quantitative approach to One Health
A major challenge for the One Health community is quantifying the interactions and risks at the human-animal-environment interface. The One Health approach recognizes the health of humans, domestic and wild animals, plants, and the wider environment are closely linked and interdependent.
Using a quantitative approach based on the One Health concept and specific structures in the network, the research confirms that, in Austria, zoonotic spillover is most likely to occur at human-cattle and human-food interfaces. “Eating contaminated food poses a major risk of human zoonotic infection, with Listeria, Salmonella, and Escherichia being the most frequently reported agents in our study,” says Desvars-Larrive.
Public awareness
“With our interactive map, we also hope to arouse curiosity and educate,” adds the epidemiologist. “We all come into contact with pathogens, but only a few cause illness, so we shouldn’t worry too much. But it is still important to develop some awareness – for example, how to prevent cross-contamination by cleaning your knife between foods.”
“Or if you have been bitten by a tick, you should be vigilant over the next few days or even weeks, as ticks transmit a whole range of diseases to humans and animals, which are often difficult to diagnose as the symptoms may not appear until weeks later,” says Desvars-Larrive.




No comments yet