Most disease-causing bacteria are known for their speed: In mere minutes, they can double their population, quickly making a person sick. But just as dangerous as this rapid growth can be a bacterium’s resting state, which helps the pathogen evade antibiotics and contributes to severe chronic infections in the lungs and blood, within wounds, and on the surfaces of medical devices.
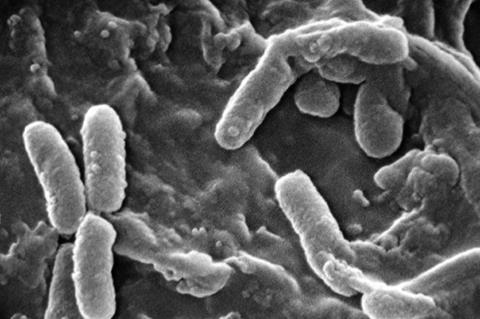
Now, Scripps Research scientists have discovered how long chains of molecules called polyphosphates (polyP) are needed for bacteria to slow down movements within cells and let them enter this resting state. The findings, published in Proceedings of the National Academy of Sciences on April 02, 2024, could eventually lead to new ways of treating chronic infections in which typical antibiotics aren’t effective.
“Many current antibiotics block bacterial growth, but bacteria spend a lot of their time not growing,” says Lisa Racki, assistant professor in the Department of Integrative Structural and Computational Biology at Scripps Research and senior author of the new paper. “We really need new and creative strategies for targeting bacteria’s slow-growing and non-growing phases.”
Energy-saving state
Researchers have long known that bacteria can survive for especially long periods of time when they stop growing, entering a dormant and energy-saving state. They also knew that when bacteria enter this resting state, they use valuable energy to produce polyP strands, which form large clumps inside their cells. But scientists had been historically unsure about the purpose of polyP.
To study polyP, Racki and her collaborators turned to Pseudomonas aeruginosa, bacteria that can cause pneumonia and blood infections in people who are hospitalized or have weakened immune systems. One of the reasons P. aeruginosa can be so hard to treat is that it forms biofilms—tightly joined, slimy communities of bacteria, many of which are in a resting state and can evade typical antibiotics.
When P. aeruginosa is starved of nitrogen—one of the key nutrients it needs for growth—it produces lots of polyP. In the new work, Racki and her collaborators at EPFL and Caltech discovered that a mutant unable to make polyP cannot enter its resting state. To better understand why this happens and the consequences, the researchers genetically engineered P. aeruginosa to make small, labeled particles that let them track how molecules within the bacteria were moving around.
Too much movement
“What we found is that when you get rid of polyP, everything in the cell moves too much,” says Racki. “The cells are partying when they should be taking a break.”
When starved of most nutrients, P. aeruginosa slows the movement of materials within its interior and stops dividing. But without nitrogen and polyP, the bacteria keep moving materials around at top-speed, become bigger, loosen their genetic material and continue dividing.
Racki’s team concluded that polyP is usually responsible for helping P. aeruginosa—and likely other bacterial species—slow down. It also leads them to hypothesize that preventing cells from producing polyP could keep them active and make them more susceptible to some antibiotics.
How things diffuse
“This not only helps point in possible directions for treating pathogenic bacteria, but also reveals answers for fundamental questions about how things diffuse throughout a bacterial cell,” says Racki.
Racki and her lab are now planning more experiments to better probe exactly why cells cannot slow their interior movements without polyP, and whether blocking the bacterial production of polyP could be an effective tactic to treat some infections.




No comments yet