Bacteria are constantly engaged in a struggle for survival, facing threats from immune cells, antibiotics, or phages— viruses that only infect bacteria. Over the course of evolution, bacteria have developed numerous strategies to protect themselves from such dangers. But how do bacteria sense potential threats in their environment and initiate protective measures?
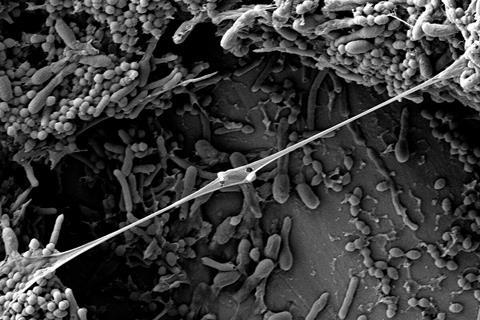
In their recent study, published in the journal Nature Microbiology, researchers led by Prof. Knut Drescher at the Biozentrum, University of Basel, have discovered that fragments of the bacterial cell wall, so-called peptidoglycans, serve as an alarm signal indicating danger in the environment.
“These molecules act as a general danger signal recognized not only by conspecifics but also by bacteria of different species,” says Drescher “Peptidoglycans are released when bacteria are killed by phages or antibiotics.”
Biofilm formation
Bacteria respond to this danger signal by producing a small signaling molecule known as c-di-GMP, which triggers biofilm formation. Biofilms are complex, three-dimensional structures of living bacteria embedded in a slimy matrix. “In Vibrio cholerae, the cholera-causing pathogen, even a brief exposure to cell wall fragments triggers biofilm formation,” explains Sanika Vaidya, first author of the study. Within the biofilm, bacteria are protected from attacks by phages, immune cells, and antibiotics.
READ MORE: The geometry of life: Physicists determine what controls biofilm growth
READ MORE: Your Pseudomonas aeruginosa biofilm may vary - depending on where it turns up
Survival strategy: Cross-species warning
The researchers observed this behavior not only in the cholera pathogen but also in other dangerous, often multi-drug resistant pathogens such as Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, and Enterococcus faecalis.
The fact that bacteria across species respond to the same danger signal suggests a universal survival strategy. “Interestingly, human immune cells also recognize peptidoglycan fragments as an infection signal,” emphasizes Drescher. “This highlights surprising parallels between bacterial and human defense mechanisms.”
This universal survival strategy may explain why biofilms play such an important role in various environments — from natural ecosystems to human infections. However, the study raises new questions: Do the cell wall fragments activate additional protective mechanisms beyond biofilm formation? And how can these novel insights be applied to more effectively combat biofilm-forming pathogens?
Topics
- Acinetobacter baumannii
- Bacteria
- biofilm-forming pathogens
- Biofilms
- Enterococcus faecalis
- Infection Prevention & Control
- Knut Drescher
- multi-drug resistant pathogens
- One Health
- peptidoglycans
- Pseudomonas aeruginosa
- Research News
- Sanika Vaidya
- Staphylococcus aureus
- UK & Rest of Europe
- University of Basel
- Vibrio cholerae







No comments yet