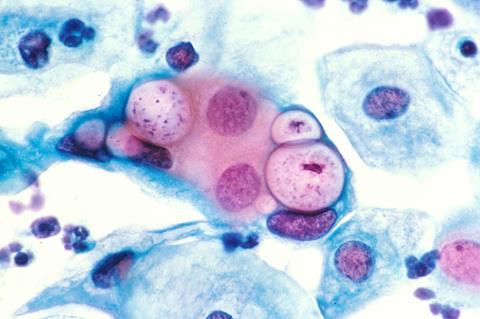
Bacteria that cause diseases, so-called pathogens, develop various strategies to exploit human cells as hosts to their own advantage. Together with medical professionals and experts for structure determination and imaging, a team of biologists from Heinrich Heine University Düsseldorf (HHU) has uncovered the attack strategies employed by the bacterium Chlamydia pneumoniae (for short: C. pneumoniae).
In the scientific journal Nature Communications, they describe which molecular mechanisms the bacterium utilises.
Chlamydia infect human and animal host cells. C. pneumoniae, for example, is transmitted via droplet infection and attacks the respiratory tract, causing bronchitis, asthma or pneumonia. The pathogens are however also linked with secondary conditions such as Alzheimer’s disease, Reiter’s disease, arteriosclerosis and lung cancer.
READ MORE: Sphingomyelins (TFSM) can visualise Chlamydia inclusions within infected human cells
READ MORE: Chlamydia can form reservoir in the intestine
At HHU, the research group headed by Senior Professor Dr Johannes H. Hegemann at the Institute for Functional Microbial Genomics has examined the infection mechanisms of the bacterium together with the Center for Structural Studies (CSS), the Center for Advanced Imaging (CAi) and the Institute of Biochemistry and Molecular Biology II at the Medical Faculty (research group headed by Professor Dr Reza Ahmadian). For the first time, the researchers describe the structural and functional methods C. pneumoniae uses to penetrate the human cell: It mimics molecular structures of the human cell (so-called “molecular mimicry”) and uses them for its attack.
Transport machinery
The bacterium can only reproduce inside a host cell. To achieve this, it needs to induce the transport machinery of the cell to incorporate it into the host – so-called endocytosis. In this process, the cell membrane curves inwards to surround the small pieces of material to be transported into the cell and then buds off inside the cell to form a membrane-enclosed vesicle containing the material.
The critical element in the process is the inner so-called actin cytoskeleton of the cell, which supplies the energy needed for endocytosis. The process is triggered by the human protein Cdc42 binding to a specific activator (N-WASP).
Lead author Fabienne Kocher, biology PhD student and member of the Manchot Graduate School “Molecules of Infection IV”, explains how C. pneumoniae hijacks endocytosis for its own ends: “Once the pathogen has bound to the outside of the human cell, it injects the chlamydial protein ‘SemD’ into its future host. The SemD then binds from the inside to the membrane of the vesicle which forms, activating the actin cytoskeleton so that the plasma membrane fully engulfs the large Chlamydium.”
This alters endocytosis to benefit the bacterium, as the process is not normally intended for the transport of such large structures as an entire bacterium.
Bacterial protein
Professor Hegemann, corresponding author of the study: “We wanted to know how the various molecular structures interact with each other and how the Chlamydia have developed to infect human cells as efficiently as possible. The bacterial protein SemD is in fact optimally tailored to N-WASP: The key section where it binds to N-WASP looks exactly like Cdc42 and it binds even better than the normal activator Cdc42.”
Professor Ahmadian from the Medical Faculty adds: “We were also able to show that SemD even displaces Cdc42, which has already bound to N-WASP, so it can then bind itself.”
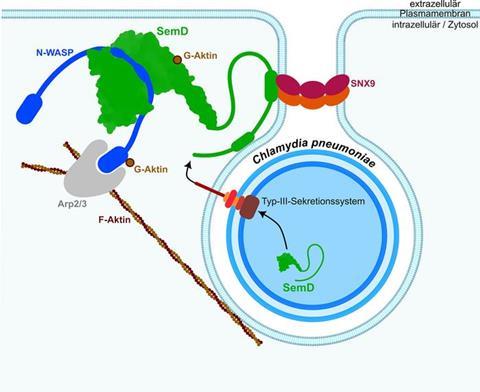
To enable determination of the structure, the researchers cultivated tiny crystals of SemD with N-WASP and then examined the structure. The team headed by Professor Dr Sander Smits at the CSS was responsible for this: “In order to realise these complex measurements, state-of-the-art technical equipment and above all corresponding personnel are needed. This concentration of infrastructural equipment and personnel expertise is not possible in every laboratory. Special centres – like the CSS established by HHU – are needed.”
Fabienne Kocher on the further perspectives: “We hope to be able to develop agents in the future, which can prevent this highly specific interaction between the bacterial and human proteins, and thus block infections by C. pneumoniae.”





No comments yet