The organisms that cause visceral leishmaniasis, a potentially deadly version of the parasitic disease that most often affects the skin to cause disfiguring disease, appear to have a secret weapon, new research suggests - they can infect non-immune cells and persist in those uncommon environments.
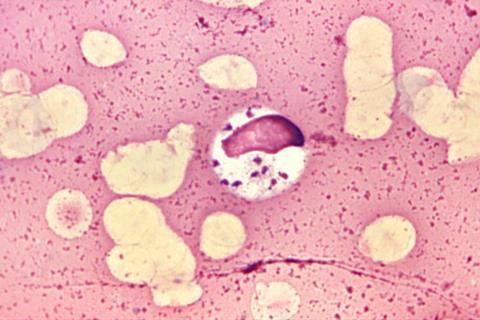
Researchers found the Leishmania donovani parasites in blood-related stem cells in the bone marrow of chronically infected mice – precursor cells that can regenerate all types of cells in the blood-forming system. The finding may help explain why some people who develop visceral leishmaniasis, which is fatal if left untreated, often also have blood disorders such as anemia.
Identifying these cells and other unexpected locations in which these parasites live improves scientists’ understanding of the disease and may lead to new treatment options, said senior study author Abhay Satoskar, professor of pathology in The Ohio State University College of Medicine.
Persistent parasites
“Treating a patient with leishmania drugs never eliminates every parasite from the body – they persist for the rest of a patient’s life,” Satoskar said. “Perhaps these uncommon cells are the cells responsible for harboring these parasites in low numbers. Some drugs may not reach these cells properly or may not be effective with those parasites, and maybe the parasites in these kinds of cells are different compared to parasites in immune cells because they can adapt. It would be important to eliminate these hidden parasites if we want to stop the transmission of the disease.
“It changes the way we think about this parasite: If uncommon cells are infected, what is the cells’ role? What are the parasites doing there? How did they evade the drug treatment? Are they different from parasites in other cells, or the same? There are lots of questions.”
The research was published recently in the journal Cell Reports.
Lingering in body
Cutaneous leishmaniasis is a disfiguring skin disease caused by Leishmania major parasites that affects up to 1.2 million people annually in the tropics, while L. donovani parasites cause the less common visceral leishmaniasis that attacks internal organs, affecting an estimated 100,000 people per year.
Scientists have suspected L. donovani may stray beyond their immune cell hosts because they linger in the body, but those suspicions have been difficult to confirm with most conventional technologies because the number of infected cells is low.
Satoskar and colleagues used single-cell RNA sequencing in their search for parasites in spleen and bone marrow cells of chronically infected mice. The technique allowed the team to identify individual cell types based on the thousands of genes expressed by cells that function as a signature of each cell type. Simultaneously, the researchers identified which types of cells were – and were not – infected by L. donovani parasites based on the presence or lack of genes known to be expressed by these organisms.
Frontline cells
In the spleen, most of the infected cells detected were frontline immune cells – macrophages and monocytes – known as phagocytes whose job is to swallow up invading organisms.
“Textbooks say Leishmania are parasites of immune cells, mainly phagocytes, that hijack those cells and live there. That is what we’ve learned for many years,” said Satoksar, also a professor of microbiology at Ohio State. “Though that was the dogma, it appears during chronic infection that they’re also infecting other cell types.”
The study showed that these infectious organisms weren’t restricted to only phagocytic cells in either organ – in bone marrow, the blood-related (hematopoietic) stem cells were the main parasitized cells, a surprising finding that was verified through a separate single-cell analysis. The fact their outer surfaces feature some of the same receptors as typical immune cell targets hints at why and how they harbor the parasites, Satoskar said.
Other types of cells in both organs whose gene expression signature suggested they contained L. donovani parasites included white blood cells that assist the immune system – but don’t engulf infectious organisms – and cells responsible for the production of platelets.
Where to look next
“Finding Leishmania genes linked to other cell signatures gives us clues of which cells to look for next in follow-up protocols,” Satoskar said.
This work has potential for rapid translation to human tissue testing in some tropical regions, where taking needle aspiration spleen and bone marrow samples is a routine procedure for people at risk for leishmaniasis. Such samples could be used to help determine if non-immune cells are occupied by parasites in humans as well.
“These are organs where these parasites persist for many, many years after an infection as cleared and a person who becomes immune suppressed can develop disease again,” Satoskar said. “With our animal data and what we find in human samples, we hope to understand the pathways that are assisting parasites to survive and use that knowledge to develop new therapies targeting those pathways.”
This work was funded by the Global Health Innovative Technology Fund and the National Institute of Allergy and Infectious Diseases.
Co-authors include Konstantinos Karagiannis, Sreenivas Gannavaram, Thalia Pacheco-Fernandez, Parna Bhattacharya and Hira Nakhasi of the Food and Drug Administration and Chaitenya Verma of Ohio State.







No comments yet