A new study in the peer-reviewed journal Human Gene Therapy indicates that DNA impurities derived from plasmid and host cell DNA are encapsulated into recombinant adeno-associated virus (rAAV) capsids as single-stranded DNA.
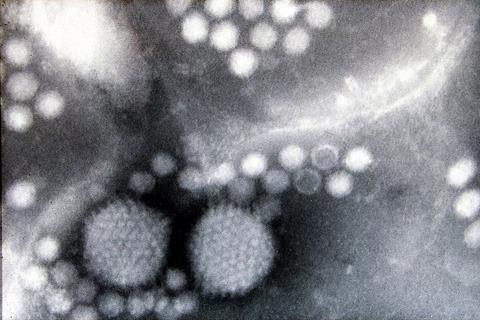
rAAVs are widely used viral vectors in human gene therapy, but contamination from DNA impurities, such as plasmid and host cell DNA, remains a significant quality control concern for their manufacture. In this study, Kazuhisa Uchida, from Kobe University, and coauthors examined several purifies rAAV samples and found that they contained DNA derived from three plasmids. They determined that the impurities were encapsulated into the rAAV capsids as single-stranded DNA.
READ MORE: New, modified CRISPR protein can fit inside virus used for gene therapy
READ MORE: Prevalence of neutralizing antibodies to AAV2 and AAV9 in individuals with Niemann-Pick disease
The investigators suggest that when rAAVs are used in gene therapy, the adverse effects of the single-stranded form of DNA impurities may differ from those of double-stranded DNA. “It may be necessary to reconsider or evaluate the effects of single-strand form of DNA impurities independently,” stated the investigators.
“Studies like this are incredibly important to better understand how DNA impurities are generated and what sources they come from during the production process in order to improve the efficiency of manufacturing and purity of the final rAAV product, which will help make the therapy more effective and reduce adverse events for patients,” says Managing Editor of Human Gene Therapy Thomas Gallagher, PhD, from the University of Massachusetts Chan Medical School.







No comments yet