Caesarean section recipients are usually given prophylactic antibiotics just before the procedure to prevent later infections at the surgical site. But there have been concerns about whether these antibiotics may have a negative impact on newborns and their microbiomes if the drugs travel through the umbilical cord and reach the baby before the cord is cut.
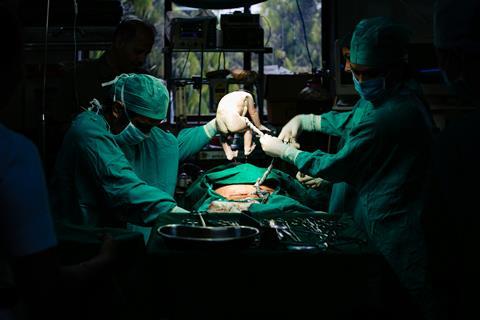
Now, a study from the Netherlands has confirmed that although these antibiotics can cause subtle changes to the infant microbiome, they are much less significant than the impact of how the babies are fed. The findings were reported August 14 in the journal Cell Host & Microbe.
READ MORE: Study reveals role of fathers in seeding the microbiota of newborns
READ MORE: Breastfeeding alters infant gut in ways that boost brain development, may improve test scores
“We decided to conduct this study because it addresses a significant clinical question with possibly profound implications for infant health,” says first and corresponding author Trishla Sinha of University Medical Center Groningen. “It is crucial to balance high-quality evidence of immediate benefits to the mother against equally robust evidence of any potential short- and long-term risks to the infant. Mothers often ask whether the antibiotics they take influence their child, and this study can provide assurance that they have only small effects on infant gut microbiome.”
Larger dataset
A handful of previous studies have looked at this question, but they had smaller sample sizes. The first part of the current study prospectively enrolled 28 mother-infant pairs. Twelve of the mothers received antibiotics before skin incision, and the other 16 received them after the umbilical cord was clamped. For this group, a total of 172 infant microbiome samples were collected at 8 different timepoints after birth, up to one year. The second part of the analysis also included data from two other similar trials for a total of 79 infants.
“Our combined analysis makes this the largest study in the field,” Sinha says. “Additionally, our longitudinal data and deep metagenomic sequencing were unprecedented.”
For the samples they collected, the researchers looked at infant gut microbiome species composition and strain variability, as well as the composition of antibiotic-resistance genes. They also looked at the composition of bile and short-chain fatty acids. In addition to information about antibiotic usage, the investigators had information about whether the infants were formula-fed or breastfed.
Feeding mode
The results showed that in general, feeding mode had a significant impact on gut microbial diversity, species, and strain-level bacterial composition, as well as bile acid composition. Infants who were formula-fed had a significantly different overall microbiome profile, with feeding mode explaining 12% of the variation in overall infant gut microbiome composition during the first six weeks of life. These differences were also reflected in the bile acid profiles in the stools of these infants.
Recent research has highlighted the crucial role of the gut microbiome and bile acids in the development of immune disorders later in life. Therefore, these early-life changes could have important long-term consequences. However, further long-term studies are needed to confirm these findings. Antibiotics, in contrast to feeding mode, only had subtle impacts on antibiotic resistance genes and strain variability.
“We were surprised that the antibiotics did not drastically alter the microbiome, because other research has reported a large impact of antibiotics on infant gut microbiome composition,” Sinha says. “This is probably due to the fact that it is a one-time exposure to intravenous antibiotics during birth in contrast to prolonged exposure to antibiotics throughout infancy.”
Lifelines NEXT
In their next study, the researchers plan to examine a group of 1,500 mother-infant pairs from the Dutch cohort called Lifelines NEXT, looking at various health, environmental, and dietary factors during pregnancy and birth, as well as factors after birth that may influence the infant gut microbiome composition. The researchers plan to follow the infants throughout childhood and into adulthood to assess the long-term impact of the infant gut microbiome on future health outcomes.
“It’s also important to realize that alterations in the gut microbiome do not immediately translate to future health outcomes for the child,” Sinha says. “These still need to be extensively studied in longitudinal studies with longer follow-up time.”





No comments yet