Toxoplasma gondii is a parasite responsible for toxoplasmosis, a lifelong chronic infection prevalent in about 30% of the human population. It poses little harm to healthy individuals but can result in severe consequences for immunocompromised people. If infection occurs during pregnancy, the parasite can cross the placenta and cause retinal or neurological issues in the developing fetus, and potentially death in severe cases.
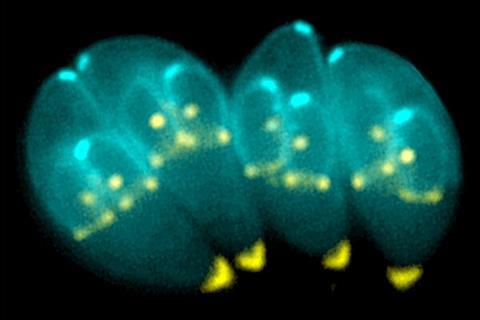
There is currently no vaccine for Toxoplasma infection, and the biological mechanism by which the parasite affects the metabolism of host cells is still understudied.
In new research published in the journal mBio, Morgridge researchers describe how Toxoplasma infection changes host cell metabolism over the course of infection using the power of optical metabolic imaging (OMI) for the first time.
Host cell metabolism
Assistant scientist Gina Gallego-Lopez, an alumna of the Morgridge Postdoctoral Fellow program and first author of the study, spearheaded this collaboration between her co-advisors, Morgridge Investigator in biomedical imaging Melissa Skala and UW–Madison professor in medical microbiology and immunology Laura Knoll.
The Knoll Lab focuses on studying T. gondii and other related parasites such as Entamoeba, while the Skala Lab develops biomedical imaging tools like OMI to monitor cellular activity.
“I have experience with parasites, but the field of optics was new for me,” Gallego-Lopez says. “I wanted to try imaging Toxoplasma gondii to see how it is affecting the metabolism of the host cell, and the best option was to use OMI because it’s non-invasive.”
Non-invasive approach
The non-invasive approach is important to be able to observe real-time changes. OMI allows researchers to monitor metabolism within live cells by detecting fluorescent activity already present in the cells. This is done without having to fix or stain samples that ultimately kill the cells.
In this study, the researchers observed changes in redox biology, measured by the chemical activity of the metabolites NAD(P)H and FAD. They found that over the course of a 48-hour infection, infected host cells became more oxidized and NAD(P)H mean lifetime increased. This result shows that the parasite can fundamentally change the metabolism of the host cell.
Additionally, they confirmed metabolic changes in the levels of glucose, lactate and ROS production — all factors associated with changes in redox biology in infected cells.
First-hand scenario
“The parasite needs energy to be able to replicate — we see those changes are different in early infection with the increase of glucose that reduces over time as well as the redox ratio in late infection,” says Gallego-Lopez. “So we have a first-hand scenario of what is happening at the level of redox biology in cells infected with Toxoplasma gondii.”
Gallego-Lopez was also curious about metabolic changes associated with the mechanism known as “kiss and spit,” where T. gondii interacts with the surface of the host cells before full invasion.
“One cell may be infected while the cells around it are not; it looks like the parasite ‘kisses’ those cells and then injects some proteins — kiss and spit,” she says. “To our surprise we were able to see similar changes as the full infection. So, it looks like a simple ‘kiss’ from the parasite is enough to induce changes in the host cell.”
Perfect tool
While there are still many questions to be answered, Gallego-Lopez says that OMI is a perfect tool to use in this scenario since it can accurately measure very small changes. After correlating OMI data and metabolomic data with gene expression analysis, the next steps include further study of genetic targets that are involved in these pathways.
“We need to go in detail — what specific gene or specific protein — to be able to design better targets for vaccine development or drug treatment but first we need to know the changes associated in the host by infection,” Gallego-Lopez says.
Ultimately, Gallego-Lopez hopes to establish her own lab to continue studying apicomplexan parasites like Toxoplasma and Cryptosporidium, the latter of which is associated with colorectal cancer.
“I want to understand how it’s possible that these parasites induce changes in the host to be able to induce cancer with time,” she says. “I’m using Toxoplasma as a model to understand the changes to later be able to apply in Cryptosporidium. That is my goal.”






No comments yet