Scientists from Duke-NUS Medical School (Duke-NUS) have developed a new approach using the Zika virus to destroy brain cancer cells and inhibit tumour growth, while sparing healthy cells. Using Zika virus vaccine candidates developed at Duke-NUS, the team discovered how these strains target rapidly proliferating cells over mature cells—making them an ideal option to target fast-growing cancerous cells in the adult brain.
Their findings, published in the Journal of Translational Medicine, potentially offer a new treatment alternative for brain cancer patients who currently have a poor prognosis.
Glioblastoma multiforme is the most common malignant brain cancer, with more than 300,000 patients diagnosed annually worldwide. Survival rates for such patients are poor (around 15 months), mainly due to high incidence of tumour recurrence and limited treatment options. For such patients, oncolytic virotherapy—or the use of engineered viruses to infect and kill cancer cells—may address the current therapeutic challenges.
Weakened viruses
Zika virus is one such option in early development. The Duke-NUS team used Zika virus live-attenuated vaccine (ZIKV-LAV) strains, which are “weakened” viruses with limited ability to infect healthy cells but can still grow rapidly and spread within a tumour mass.
“We selected Zika virus because it naturally infects rapidly multiplying cells in the brain, allowing us to reach cancer cells that are traditionally difficult to target. Our ZIKV-LAV strains also replicate themselves in brain cancer cells, making this a living therapy that can spread and attack neighbouring diseased cells,” said Dr Carla Bianca Luena Victorio, first author of the paper and Senior Research Fellow at the Cancer & Stem Cell Biology Research Programme at Duke-NUS.

Dr Victorio and the team determined that ZIKV-LAV strains were highly effective in infecting cancer cells as these viruses bind to proteins that are present in high levels only in cancer cells and not in healthy cells. Upon infecting a cancer cell, these virus strains hijack the cell’s resources to reproduce, ultimately killing the cell. As the cancer cell’s protective membrane ruptures upon death, it releases its contents, including virus progeny that can infect and kill neighbouring cancer cells. In addition, some cellular proteins released from the infected cells can activate an immune response to further inhibit tumour growth.
Tumour cells
Through their experiments, the team observed that infection from ZIKV-LAV strains caused 65 to 90 per cent of glioblastoma multiforme tumour cells to die. While the ZIKV-LAV strains also infected 9 to 20 per cent of cells from blood vessels in the brain, the infection did not kill these healthy cells. In contrast, the original parent Zika virus strain killed up to 50 per cent of healthy brain cells.
The scientists also discovered that the ZIKV-LAV strains were not able to reproduce well even when they managed to infect healthy cells. The amount of virus measured in healthy brain cells infected with ZIKV-LAV was only 0.36 to 9 times higher than before infection. In contrast, the amount of virus in brain cancer cells infected with ZIKV-LAV was 100 to a billion times higher than before infection. This further illustrates that conditions in cancer cells are significantly more conducive for virus reproduction than in normal cells.
“Since the Zika virus outbreak in 2016, understandably, there has been fear about the nature of the virus and its devastating effects. Through our work, we hope to present the Zika virus in a new light by highlighting its potential to kill cancer cells. When a live virus is attenuated, such that it is safe and effective to fight infectious diseases, it can be beneficial to human health—not just as a vaccine but also as a potent tumour-eradicating agent,” said Assistant Professor Ann-Marie Chacko from Duke-NUS’ Cancer & Stem Cell Biology Research Programme. She is also the senior author of the paper.
Screening tool
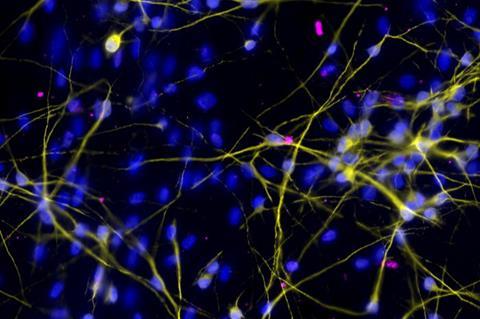
The live attenuated virus strains were originally developed as a vaccine by Professor Ooi Eng Eong’s group from Duke-NUS’ Emerging Infectious Diseases Research Programme. As a control, the virus strains were also tested on brain neurons or nerve cells that had been cultivated from human stem cells by Assistant Professor Alfred Sun’s team from the Neuroscience & Behavioural Disorders Research Programme in Duke-NUS. This provides a reliable screening tool to assess the safety and efficacy of using the virus as therapy in human cells.
Asst Prof Chacko’s group is improving these and other Zika virus strains to increase their potency in killing not only brain cancer cells, but other types of cancer cells as well, while making them safer for use in patients. They are also modifying the virus so it can be imaged non-invasively after it has been injected into a patient. This will allow doctors to monitor where the virus goes in the patient and how long it is functional in the tumour.
To this end, the group is exploring commercialising their virus strains as both a Zika vaccine and treatment for brain cancer, and potentially other cancers, such as ovarian cancer.
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, said: “This is a sterling example of how different research programmes within the School come together to tap their various expertise to advance medical knowledge and improve patients’ lives. The team’s valuable insights may one day translate into a new treatment option to control tumour growth or even, offer a cure for cancer.”







No comments yet