Scientists led by the Institute of Nanotechnology in Italy, in collaboration with the ESRF, the European Synchrotron in Grenoble, France, have discovered how X-ray micro- and nano- tomography can provide clues on the processes that link the gut neurons with those in the brain and may trigger Alzheimer’s. The results are published in Science Advances.
Alzheimer’s disease, the most common type of dementia, is a neurodegenerative disorder characterized by brain alteration including synaptic loss, chronic inflammation and neuronal cell death.
In recent years, scientists have found evidence that the gut and the brain communicate through the neurons placed in both organs. Dysfunction in this axis has been linked to psychiatric and neurological disorders, including Alzheimer’s.
Gut microbiota
The gut microbiota, which refers to the microorganisms in the intestinal tract, plays a key role in human health and influences brain function, cognition and behaviour. “There are already many studies that support that changes in the gut composition can contribute to Alzheimer’s onset and progression,” explains Alessia Cedola, researcher from the Institute of Nanotechnology in Italy and corresponding author of the article.
READ MORE: Researchers uncover new link between gut bacteria and Alzheimer’s disease
READ MORE: Researchers use AI to improve Alzheimer’s treatment through the ‘gut-brain axis’
In particular, dysbiosis, which is the process by which there is a loss of microbial diversity, induces the prevalence of dangerous bacteria producing toxic metabolites promoting inflammation, and, consequently, the breakage of the gut/brain barriers.
What happens exactly when gut dysbiosis occurs? “The main hypothesis is that changes trigger the escape of bad bacteria from the gut, entering the circulation, reaching the brain and triggering Alzheimer’s, but evidence is still poor,” adds Cedola.
Getting to the core of gut health
Now scientists have discovered that nano- and micro X-ray phase-contrast tomography (XPCT) is a powerful tool to study structural and morphological alterations in the gut, without tissue manipulation. The team came to the ESRF, the European Synchrotron, in Grenoble, France, to scan samples on beamline ID16A.
“Thanks to this technique we can image soft biological tissues with excellent sensitivity in 3D, with minimal sample preparation and without contrast agents,” explains Peter Cloetens, scientist in charge of ID16A and co-author of the publication.
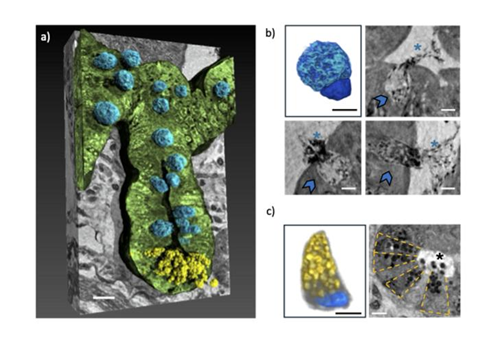
The data of the experiments, partially carried out also at Soleil, showed the changes in cell abundance and organisation in the tissues, as well as structural alteration in different tissues of mice affected with Alzheimer’s.
Specifically, it showed relevant alterations in the villi and crypts of the gut, cellular transformations in Paneth and goblet cells, along with the detection of telocytes, neurons, erythrocytes, and mucus secretion by goblet cells within the gut cavity. All these elements, when working correctly, maintain gut health, support digestion, and protect the intestinal lining from damage.
Breakthrough for gut analysis
“This technique represents a real breakthrough for the thorough analysis of the gut, and it could be pivotal in early detection and prognosis of the disease,” says Cedola.
And she adds: “As a long-time user of the ESRF, I can attest to the incredible opportunities that this facility provides for cutting-edge research, and the nanoimaging beamline, especially with the EBS. Coming to the ESRF has been instrumental in advancing our understanding of the gut-brain axis in Alzheimer’s disease.”
Together with scientists Francesca Palermo and Claudia Balducci, the next steps in this research will be to further exploit the capabilities of XPCT to study how the gut communicates with the central nervous system. The team aims to investigate the enteric nervous system and its role in Alzheimer’s disease.
New targets and therapeutics
“By gaining a deeper understanding of these processes, we hope to identify new therapeutic targets and develop innovative treatments for this devastating disease. The ESRF will undoubtedly continue to play a crucial role in our research, and we look forward to many more exciting discoveries in the years to come,” concludes Cedola.
This research highlights the importance of biomedical studies at the ESRF, as the facility aims to further enhance this focus in the coming years.







No comments yet