A new study from the Faculty of Medicine at the Hebrew University of Jerusalem sheds light on how bacterial motion influences the spread of antibiotic resistance. Led by Professor Sigal Ben-Yehuda and Professor Ilan Rosenshine from the Department of Microbiology and Molecular Genetics, the research uncovers a direct connection between the rotation of bacterial flagella—structures used for movement—and the activation of genes that enable bacteria to transfer DNA to one another.
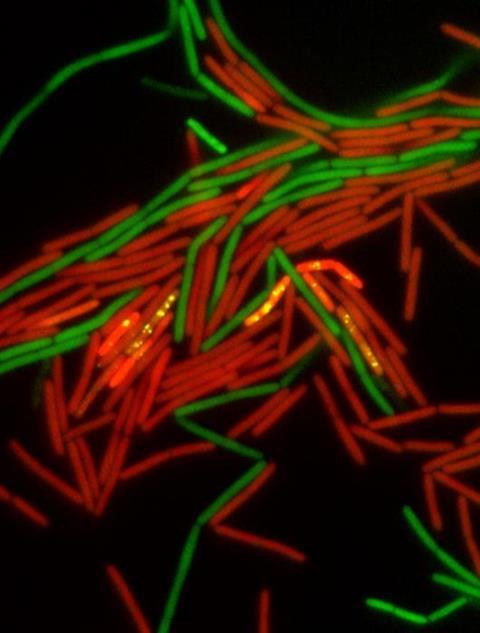
This process, known as bacterial conjugation, is a key mechanism by which genetic traits, particularly antibiotic resistance, are shared among bacterial populations. While conjugation has traditionally been associated with bacteria attaching to solid surfaces, the team investigated pLS20, a widespread conjugative plasmid in Bacilli species, which behaves differently. The study shows that in liquid environments, where bacteria rely on movement to navigate, the rotation of flagella acts as a mechanical signal that turns on a set of genes required for DNA transfer.
Triggering gene expression
The researchers discovered that this signal triggers gene expression in a specific subset of donor cells, which then form clusters with recipient bacteria.
READ MORE: Researchers have uncovered how foreign DNA can evade bacterial defense systems and neutralize them
READ MORE: Researchers shed light on the mechanisms of bacterial flagellar motors
These multicellular clusters bring the two types of cells into close contact, facilitating the transfer of genetic material. Importantly, the study demonstrates that it is not just the presence of flagella, but their ability to rotate, that is critical. When flagellar rotation was disrupted—either genetically or by increasing the viscosity of the surrounding medium—conjugation activity declined significantly. This suggests that bacterial motility is not only important for movement but also serves as a mechano-sensing signal for genetic exchange.
“Our study brings about a novel notion that synchronizing DNA transfer with the bacterial motile lifestyle provides the plasmid with the advantage of spreading into remote ecological niches,” said Prof. Sigal Ben-Yehuda
These findings provide valuable insight into how mobile genetic elements synchronize with host physiology to enhance their own transmission. Understanding these mechanisms may help in developing new strategies to limit the spread of antibiotic resistance—a major public health concern.






No comments yet