This study, published in mLife, was led by Prof. Ming Li and Prof. Hua Xiang (Institute of Microbiology, Chinese Academy of Sciences). Early bioinformatic analyses revealed that, in haloarchaeal genomes, the ago genes are frequently accompanied by genes encoding proteins with an N‐terminal PLD family domain, which designated agaP (encoding AgaP) genes in this study.
To fully understand the evolutionary link between ago and agaP genes in haloarachaea, a systematic investigation of available haloarchaeal genomes in NCBI was conducted. The analysis revealed that ~9.6% (63 out of 656) of haloarchaeal species have ago genes, of which ~44.4% (28 out of 63) are associated with AgaPs.
The agaP genes can locate upstream or downstream of their associated ago genes, usually with a short intergenic region ranging from 31 to 64 bp. Further characterization of the transcription pattern of the ago‐agaP operon from Natrinema pellirubrum revealed that they are co-transcribed, strongly indicating functional coupling between them.
Knock-in mutants
When transforming the knock-in mutants with empty vector, the transformation efficiency of ago+/agaP+ cells was reduced by ~82% compared to that of wild type (WT). Moreover, the ago+/agaP+ cells formed colonies that were noticeably smaller than those of WT. Therefore, the co‐expression of N. pellirubrum Ago and AgaP together in H. hispanica cells restricts plasmid transformation. Subsequently, these colonies were inoculated into a selective liquid medium (to maintain plasmid) and cultivated until they reached a similar cell concentration.
Following serial dilution, these cell cultures were separately spotted onto selective and nonselective plates. Interestingly, the results showed that the pAgo system not only limited plasmid transformation but also effectively expelled the intracellular plasmid DNA under nonselective conditions. In addition, mutational analysis revealed that the catalytic activity of AgaP is essential for the observed plasmid resistance.
Viral infection
Viral infection assay showed that the ago+/agaP+ mutant exhibited a significant reduction (approximately 90%) in plaque-forming units (PFUs) compared to the WT strain, demonstrating the virus immunity conferred by NpAgo-PLD (APAP) system. Intriguingly, the expression of only the pAgo protein provides a lower level of virus immunity. A closer examination of the plaques revealed that the plaques formed on the lawn of ago+/agaP+ cells were significantly smaller compared to those formed on the lawns of WT, ago+, or ago+/agaPM cells.
Thus, the PLD protein and its catalytic activity are important, although may be not essential, for the immunity against HHPV-2. Upon evaluating the virus susceptibility of agoM/agaP+ cells, a high level of PFU was observed, highlighting the critical role of the catalytic center of NpAgo during the immunity against the virus. These data demonstrated the catalytic activity of NpAgos is important for resisting single-stranded DNA virus, and the PLD proteins play an auxiliary role in enhancing immunity against virus.
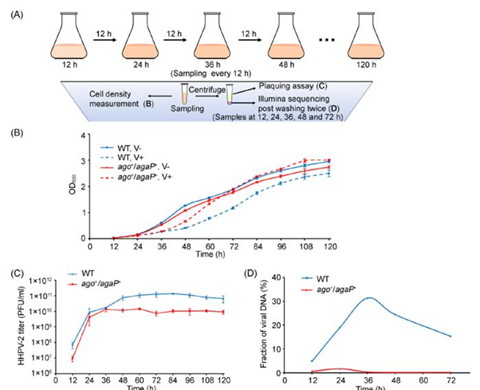
HHPV‐2 infection caused a noticeable retardation in the growth curve of both WT and ago+/agaP+ strains. However, after 36 hours post infection (hpi), the growth curve of ago+/agaP+ started to recover more quickly than that of WT (Fig. 1). These findings suggest that the N. pellirubrum APAP system starts to produce active immunity against HHPV‐2 before 36 hpi without inducing cell death/dormancy. Therefore, this auxiliary PLD-associated pAgo system does not employ the Abi strategy. Consistently, subsequent Illumina sequencing data demonstrate that the APAP system effectively suppresses the propagation of viral DNA in H. hispanica cells and, as a result, significantly reduces the production of viral progenies (Fig. 1).
Potential in genome editing
One of the exciting aspects of this study is the potential application of NpAgo system in genome editing. When expressed in E. coli, NpAgo protein enhanced gene editing efficiency, though this effect was surprisingly dampened by the presence of PLD proteins. This finding suggests that the pAgo-PLD system could serve as a versatile tool for gene editing, with fine-tuned regulation of PLD activity, providing a way to manage editing effects or enhance efficiency.
The study highlights the ability of catalytically active pAgo proteins to employ auxiliary proteins to strengthen the targeted eradication of different genetic invaders and underline the trend of PLD nucleases to participate in host immunity.





No comments yet