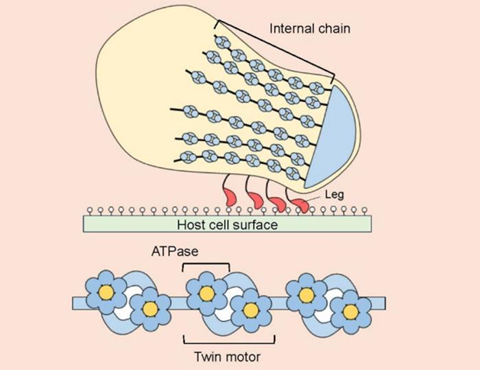
Mycoplasmas, including bacteria that cause pneumonia in humans, are generally nonmotile, but Mycoplasma mobile, as the species name suggests, has been found in the gills of fish and seems to move by gliding along surfaces. The molecular structure that allows it to do so has for the first time been uncovered by a collaborative research group led by Osaka Metropolitan University Professor Makoto Miyata of the Graduate School of Science.

The OMU-led research team has been working since 1997 to clarify M. mobile’s motility mechanisms. This time, with the help of Osaka University’s cryo-electron microscopy equipment, the scientists revealed at near-atomic resolution the enzymes known as ATPases that use rotational catalytic mechanisms to power the gliding machinery.
In addition, although the molecular structures of the two units that make up the twin motor are similar to those of known ATP synthases, the researchers found that they combine to form an unprecedented complex structure.
“This achievement will promote further understanding of the motility mechanism of how the energy of ATP hydrolysis is converted into gliding motility, and how these motors of Mycoplasma mobile are thought to have evolved from ATP synthase,” Professor Miyata explained. “Going forward, we can expect our results to be used as a basis for applications in nanobot actuators and for the development of medicines to fight mycoplasma infections.”




No comments yet