A recent study has developed nanovesicles (NVs) from activated neutrophils, showcasing their ability to perform molecular debridement and accelerate healing in infectious wounds. This novel method significantly enhances treatment effectiveness, particularly for stubborn diabetic wounds, by targeting and neutralizing deep tissue pathogens.
Infectious wounds represent a critical challenge in healthcare, especially for diabetic patients grappling with ineffective antibiotics and escalating drug resistance. Conventional therapies often inadequately address deep tissue infections, highlighting the need for more innovative solutions.
READ MORE: Revolutionary chronic wound treatment could help millions
READ MORE: Orangutan treats wound with antimicrobial, pain-relieving plant
Engineered nanovesicles (NVs) from activated neutrophils provide a precise mechanism to combat pathogens deeply embedded in tissues, potentially revolutionizing the management of complex infectious wounds and boosting overall treatment efficacy.
Significant advance
Researchers at the Research Center for Neutrophil Engineering Technology have achieved a significant advancement in medical nanotechnology. Their findings (DOI: 10.1093/burnst/tkae018), published in the journal Burns & Trauma on June 20, 2024, detail the creation of novel neutrophil-engineered NVs. These NVs, rich in bactericidal proteins, are set to transform infectious wound treatment by amplifying the body’s natural immune response at the injury site.
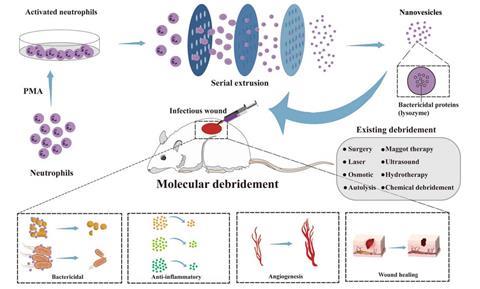
This study reveals that engineered NVs derived from activated neutrophils not only mimic the physical properties of exosomes but surpass them due to their rich content of bactericidal proteins. Extensively tested both in vitro and in vivo, these NVs effectively combat key pathogens like Staphylococcus aureus and Escherichia coli, which contribute to deep tissue infections. The NVs promote rapid debridement, significantly reduce bacterial populations, and boost collagen deposition, thus hastening the healing process. This research positions NVs as a formidable alternative to traditional antibiotics, introducing a novel method for treating resistant infections and advancing the field of wound care.
Bactericidal proteins
Dr. Bingwei Sun, the lead researcher, said: “These engineered NVs mark a major advancement in the management of infectious diseases. By targeting the infection site with high levels of bactericidal proteins, we achieve swift and effective healing, thereby opening new paths for the treatment of chronic and resistant infections.”
The advent of activated neutrophil-derived NVs signifies a major leap in medical technology, potentially reducing healthcare costs and enhancing patient outcomes. This innovation not only promises to improve wound healing in diabetic and other chronic infection patients but also sets the stage for further development of biologically inspired therapeutic strategies.







No comments yet