Researchers have discovered that a key bacterial protein, CsrA, gathers in a droplet-like structure inside cells to control when and how bacteria activate their disease-causing genes. This newly identified compartment, which forms without a membrane, acts as a kind of temporary control center, helping bacteria adapt to their environment and switch between being harmless and virulent—offering new insight into how infections begin and how they might be stopped.
Scientists at the Hebrew University of Jerusalem have uncovered a surprising way in which harmful bacteria prepare to attack their hosts. The discovery, led by PhD. students Lior Aroeti, Netanel Elbaz under the guidance of Prof. Ilan Rosenshine from the Faculty of Medicine could one day help researchers find new ways to fight infectious diseases.
READ MORE: A lung pathogen’s dilemma: infect or resist antibiotics?
READ MORE: Molecular switch plays central role in bacterial dysentery
At the heart of this study is a protein called CsrA, which acts like a switchboard operator inside bacterial cells. It helps bacteria decide which of their genes to turn on or off—especially the genes that make them dangerous to humans.
Membraneless compartment
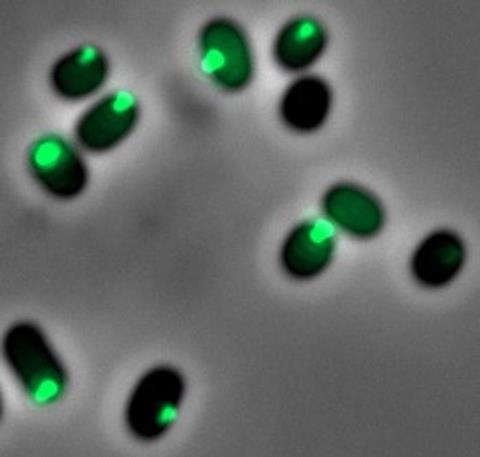
Researchers have long known that CsrA plays a central role in bacterial virulence—the ability of bacteria to cause disease. But the new study shows that CsrA doesn’t work alone. Instead, it gathers in a special, droplet-like structure inside the cell. This structure has no membrane, making it a “membraneless compartment,” which scientists now believe is crucial in regulating how bacteria behave.
“This compartment acts like a temporary control center,” explained Prof. Rosenshine. “It helps bacteria shift gears—either gearing up to infect or slowing down to conserve energy.”
Using glowing proteins to track CsrA inside bacteria, the team discovered that these compartments form naturally under certain conditions, especially those that mimic the human gut. They also found that these structures include other key molecules that help bacteria manage their genetic activity.
New window
One of the most exciting findings is that these compartments might be common across many different kinds of bacteria—not just one species. This could mean that similar mechanisms are being used by a wide range of pathogens to control how and when they become dangerous.
Prof. Rosenshine’s research provides a new window into how bacterial cells are organized and how they adapt to their environment. Understanding this could pave the way for future treatments that stop bacteria from becoming harmful in the first place.






No comments yet