Unlike plants and cyanobacteria, photosynthetic bacteria, such as purple sulfur bacteria, thrive in extreme environments with high salt concentrations and alkalinity. These bacteria use hydrogen sulfide (H2S) to convert solar into chemical energy.
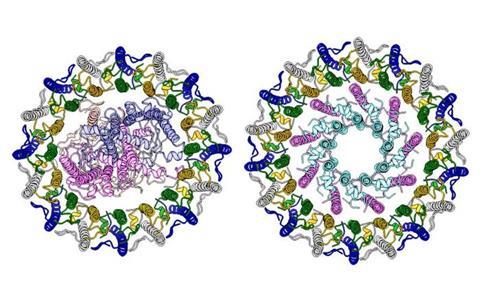
Light-harvesting protein complexes—specifically the light-harvesting two complex (LH2) and the core light-harvesting reaction center complex (LH1-RC)—play a crucial role in this process. Halorhodospira halophila, a purple sulfur bacterium, is believed to perform photosynthesis efficiently by integrating LH2 and LH1-RC. However, in nonsulfur bacteria, the interaction between LH2 and LH1-RC has been reported to be weak, and this key difference remains unclear.
Amino acid level
To investigate this, researchers employed cryo-electron microscopy to observe LH2 and LH1-RC from Hlr. halophila at the amino acid level. Results revealed that LH1-LH2 and LH1-RC complexes are formed, the smallest unit of the LH1 structure is composed of an unusual polypeptide chain, and this LH1 structure surrounds LH2 or RC. Furthermore, experiments measuring intermolecular energy transfer showed that the LH1-LH2 complex achieves almost 100% light energy transfer efficiency, suggesting that its structural arrangement enhances energy conversion.
READ MORE: The hunt for the halophilic soda-lake vampire
READ MORE: Game-changing purple bacteria can become bioplastic factories
These findings provide new insights into how bacteria perform highly efficient photosynthesis even under extreme conditions while converting toxic H2S into sulfur. This knowledge could contribute to advancements in solar energy and environmental conservation.




No comments yet