Seeps on the deep seafloor naturally emit alkanes, which are pollutants that are potentially dangerous to life and act on global warming. Fortunately, the sediments around the seeps host microbes that act as a biological filter: They consume most of the alkanes before their release into the oceans and our atmosphere.
This so-called anaerobic oxidation of alkanes is an important yet poorly understood microbial process. Scientists from the Max Planck Institute for Marine Microbiology in Bremen, Germany, now present a study on the degradation of ethane, the second most abundant alkane in seeps. They characterized enzymes involved in the process and found that their reaction breaks an established dogma in the field of anaerobic biochemistry. Their results are published in Nature Communications.
READ MORE: Efflux pumps conferring antibiotic resistance found in archaea for the first time
READ MORE: Scientists unlock secrets of how the third form of life makes energy
The anaerobic oxidation of ethane was described a few years ago, and many of its secrets still have to be unraveled. “When drawing the chemical reactions of the pathway on paper, we found large gaps of uncharted biochemistry. We deduced that the involved organisms must acquire cellular energy through an unknown path,” explains first author Olivier Lemaire.
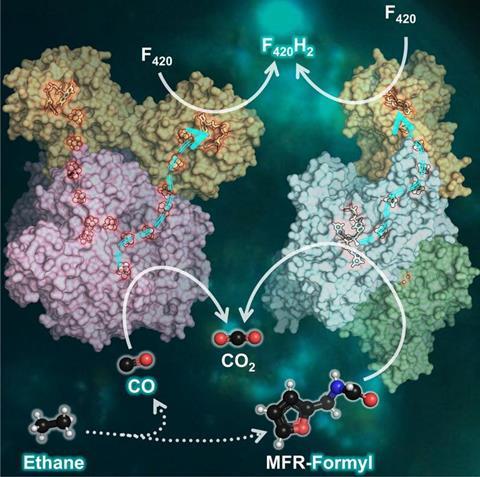
The two last enzymes of the process generate carbon dioxide (CO2) from the ethane. Other microbes use a protein called ferredoxin to take up electrons produced along the way. “That was also assumed in ethane oxidizers. However, when we looked at the genome of the microbes, we found that they don’t have the enzymatic tools to obtain cellular energy by the use of ferredoxin. Thus, something else must be at play.”
A challenging study
Solving this riddle was only possible thanks to a close collaboration within the Max Planck Institute for Marine Microbiology. Gunter Wegener and his team sampled the ethane-degrading microbial consortium from the deep sea and managed to culture it in the lab, despite this being a very demanding task. Using these cultures, the group of Tristan Wagner managed to isolate and characterise the enzymes involved in ethane oxidation.
“Isolating enzymes from such a precious and complex microbial culture is a real challenge, but we managed with a lot of effort and meticulousness,” says Tristan Wagner.
The analyses now published show that both enzymes harbor an additional protein, electronically connected to the rest of the enzyme through a wire made of iron and sulfur. This subunit allows the use of an alternative electron acceptor: The F420, a molecule based on flavin, which is a class of chemicals also important to humans (as vitamin B2, for example).
“Such assemblies of CO2-forming enzymes and F420-reductases were never before described or suspected,” says Tristan Wagner. The researchers confirmed by additional experiments that both enzymes used F420 as an electron acceptor. “This discovery breaks a dogma in the scientific field of anaerobic metabolism, as it expands what these enzymes can do.”
“We suppose that the coupling of CO2-generation with F420 as electron acceptor might stimulate the entire process. The electrons are then transferred across the cell membrane to another microbe, reducing sulfate, which is a common principle in alkane-oxidizing consortia” says Gunter Wegener.
Ethane degradation
By elucidating this metabolic riddle, Lemaire and his colleagues reveal a key aspect of the ethane-degrading microbes, which play an important role in the carbon cycle. It also shows that the knowledge gained from a few model organisms cannot be simply transposed to related species and that the enzymes involved can be more versatile than assumed.
”Our study illustrates how little we know about the metabolism of these microbes, which have lived on our planet for billions of years and can adapt to so many environments, and how important it is to understand them via experimental means,” Wagner concludes.
The study has a far-reaching impact as the alkane oxidation process performed by this type of microorganism is a crucial element of the biological filter existing in marine seeps, preventing massive effluxes of naturally produced alkanes in the atmosphere and seawater.







No comments yet