The human gut is a microbial metropolis – home to trillions of bacteria, fungi, and viruses that impact nearly every aspect of our health and well-being. Recently, these microbes (termed the “gut microbiome”) have been tied to our reproductive health through their ability to produce, break down, and modify sex hormones.
A historical perspective: how the gut-hormone link was discovered
The idea that the gut and reproductive health could be connected comes from historical times. Endometriosis, a common condition characterized by hormone imbalances and infertility, was historically named “Hysteria of the stomach” and linked to intestinal discomfort in the Middle Ages.
A stronger connection began to emerge in the 1980s. Following reports that women taking both oral contraceptives and antimicrobials reported higher rates of contraceptive failure, Adlercreutz et al. found that women taking antibiotics had lower estrogen levels. The gut microbiome, a highly dynamic collection of different microbes that reside in our intestines and are closely linked to our health, is severely altered by antibiotics. This research provided one of the first links between the gut microbiome and hormones.
A growing interest in exploring the gut-hormone connection led to an increase in studies identifying and characterizing enzymes produced by microbes that could modify hormone function. For example, several groups found that gut microbes such as Clostridium are capable of producing androgens (male sex hormones) from glucocorticoids – hormones produced in the adrenal glands. Eventually, Lyte and colleagues demonstrated that some bacteria are capable of utilizing hormones as food sources, potentially pointing to their role in depleting systemic hormone levels. From these studies, the term ’microbial endocrinology’ was coined, describing the study of microorganisms and their ability to recognize, modify, and produce hormones.
By the 2000s, the field was exploding with studies connecting microbial hormone metabolism genes with hormone-related disease states (such as polycystic ovary syndrome (PCOS), breast cancer in women, and prostate cancer in men). Novel sequencing technologies revolutionized our understanding of the gut microbiome, allowing scientists to profile how communities of bacteria impact hormones on a more holistic level. These tools allow researchers to interrogate not only the identities of the vast array of microbes in the gut, but also which genes are activated, and which enzymes are produced during various disease states. With an increasing understanding of the links between the microbiome and hormonal health, recent studies are examining the effects of probiotics on sex hormone regulation and the treatment of hormone-related diseases, with the goal of incorporating these therapies in personalized therapeutic regimens.
Changes to the gut microbiome affect reproductive health via sex hormones
Sex hormones such as estrogen, progesterone, and testosterone play a central role in regulating reproductive health and beyond. Their production, circulation, and regulation (see Figure 1) are tightly controlled by the body, with disruptions in hormone levels being closely associated with a variety of diseases and symptoms. These include depression, sexual dysfunction, decreased libido, and disorders such as endometriosis and PCOS that can affect quality of life and sexual satisfaction. Additionally, there are several cancers whose progression and treatment are sensitive to hormone levels.
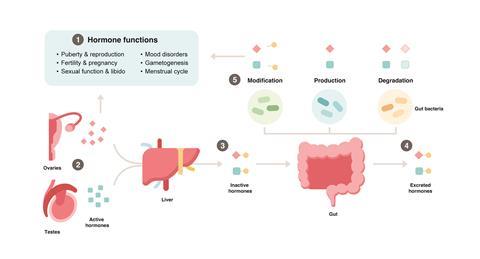
Over the years, it has become increasingly evident that the gut microbiome affects reproductive health by changing the delicate balance of hormones in the body. Three main mechanisms have been discovered that underlie the interaction between the microbiome and hormones: i) their production, ii) degradation, or iii) modification of activity by gut bacteria.
i) Production of sex hormones
Various diseases associated with the reproductive system have been linked with elevated hormone levels. For example, PCOS is a common hormonal disorder associated with higher-than-normal levels of androgens, or “male” hormones, in females. These elevated androgen levels can cause disruptions in the menstrual cycle, and ovulation, and lead to irregular periods, infertility, acne, excessive hair growth, and ovarian cysts. In humans, it was found that members of the Clostridium genus (discussed above) are linked to increased testosterone levels and can directly produce it. These species (along with several others) have been implicated in the progression of various hormone-sensitive cancers, such as prostate cancer. Furthermore, the production of estradiol from estrone (a sex hormone associated with menopause) has also been demonstrated in several gut microbiome species such as Pseudomonas aeruginosa, Staphylococcus aureus, and Bacteroides fragilis. Increased levels of estradiol in post-menopausal women are linked to breast cancer, and conversely, insufficient levels are linked to infertility.
In addition, the hormone progesterone plays a key role during pregnancy and in regulating the menstrual cycle. Recently, one study has shown that the species Gordonibacter pamelaeae and Eggerthella lenta are able to produce progestins (a synthetic form of progesterone) from glucocorticoids. Reduction in progesterone levels is also linked with infertility.
ii) Degradation of sex hormones
As discussed above, hormone deficiencies have major impacts on reproductive health. Some microbes can degrade sex hormones and reduce their systemic levels. For example, Steroidibacter denitrificans and Comamonas testosteroni are two strains that are capable of utilizing testosterone as an energy source, which can lead to sexual dysfunction and depression. Interestingly, recent studies by Li et al. have demonstrated the clinical relevance of microbiome hormone degradation in the context of depression, known to be a hormone-driven disease. They identified Mycobacterium neoaurum and Klebsiella aerogenes as two strains capable of degrading hormones that are enriched in male and female patients with depression, respectively. In animal models, when these strains were administered, the animals exhibited depression-like behaviour associated with decreased levels of testosterone and estradiol.
iii) Modification of sex hormone activity: activation and inactivation
In addition to directly affecting hormone levels by producing or breaking down hormones, hormone activity can also be affected by small changes in their structure. Specifically, when modified to contain certain chemical groups, hormones may be inactivated and secreted out of the body. Conversely, the removal of these chemical groups keeps them in circulation, in an active state.
Importantly, gut bacteria can perform these activation and inactivation reactions. For example, Bacteroides thetaiotaomicron (a highly prevalent member of the gut microbiome) encodes enzymes that can either add or remove chemical groups to inactivate or activate sex hormones, respectively. Many studies have investigated this in the context of estrogen-driven diseases (e.g. endometriosis, PCOS, and breast, ovarian, and endometrial cancers). In these conditions, changes in bacterial levels are linked to altered active hormone levels and disease states.
The future of gut-hormone research
Recent discoveries such as the ones presented above have helped us to build a more complete picture of how gut microbes affect sex hormones. Even now, androgen-producing microbes are being studied for their role in potentially inhibiting prostate cancer therapies – and methods of depleting them are being explored. Additionally, probiotic-induced regulation of estrogen has been suggested to improve symptoms of PCOS and menopause. Overall, by gaining a better understanding of how gut microbes can affect sex hormone levels, the field has now moved towards designing personalized therapeutic strategies and exciting new ways to target each person’s unique microbiome and hormonal landscape to improve reproductive health.








No comments yet