Norovirus, also known in popular media as the ‘winter vomiting bug’ or ‘two-bucket winter bug’, is a highly infectious non-enveloped RNA virus that is a major cause of acute and chronic gastroenteritis. Globally, nearly 700 million cases are attributed to norovirus annually, resulting in approximately 200,000 deaths, with the majority of fatalities in children under the age of five and adults over the age of 65.
In the UK, norovirus causes an estimated 3 million cases annually, costing the NHS £108 million with a total economic burden of nearly £298 million. Alarmingly, recent UKHSA figures indicate that norovirus cases are at their highest in five years. A greater proportion of mortalities also occur in lower-income countries due to severe diarrhea and dehydration, and in the absence of a vaccine, prevention of norovirus infections is critical.
Norovirus is a small non-enveloped RNA virus, belonging to the Caliciviridae family of viruses. The mature virus particle, or virion, consists of the positive-sense, single-stranded RNA genome enclosed in an icosahedral capsid, typically measuring 23-40 nm in diameter, and composed of major capsid viral protein (VP1) and minor capsid viral protein (VP2). As expected for RNA viruses, norovirus exhibits vast genetic diversity; there are ten genogroups (GI-GX) and 49 genotypes of norovirus, as determined by the sequence of VP1. A number of variants of norovirus have also been associated with pandemics and sporadic outbreaks of acute gastroenteritis, of which GII.4 Sydney 2012 is the predominant strain worldwide, although rapid evolution results in new GII.4 epidemic strains every two to three years.
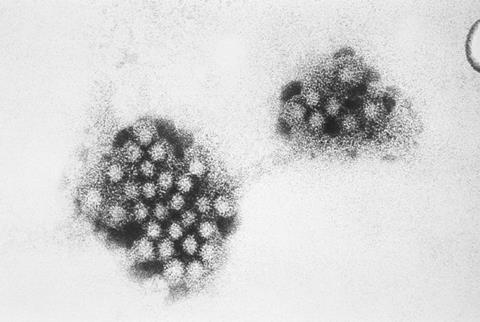
Infection occurs primarily through the faecal-oral route, where infectious particles are dispersed in droplets during vomiting or diarrhea. These can then deposit on surfaces as fomites to cause infection. Norovirus particles are particularly environmentally resistant and have been shown to remain infectious for up to two weeks on most surfaces. The norovirus capsid demonstrated stability across a wide pH range and at temperatures up to 60°C. It has been suggested that these environmental factors induce structural rearrangements in the capsid, in turn contributing to higher adsorption to surfaces. Coupled with the fact that infected individuals can rapidly produce vast quantities of virus for several days after symptoms have cleared (up to 109 particles/g feaces, detected up to 60 days), rapid transmission can easily occur in confined areas such as hospital wards, care homes, and cruise ships. The disease is self-limiting in most individuals, but chronically infected immunocompromised individuals have been observed to shed virus for months to over a year.
Therefore, the key to preventing infections is minimising viral spread by non-pharmaceutical interventions such as hand hygiene, isolation and quarantining, and disinfection of contaminated surfaces to prevent fomite transmission. Traditional disinfectants such as quaternary ammonium compounds (QACs) and alcohol are ineffective against non-enveloped viruses. Instead, chlorine-based compounds such as bleach (0.1% w/v sodium hypochlorite) are recommended for disinfection of norovirus. More recently, automated decontamination devices that use vaporised hydrogen peroxide (H2O2) or hypochlorous acid are being used as ‘no touch’ environmental disinfection methods, although their efficacies are unclear. Human norovirus (hNoV) was found to survive disinfection procedures, and efficacies were greatly reduced in the presence of organic soiling, necessitating precleaning steps or repeated disinfection cycles to be performed. Increasing the concentration of the disinfectants will eventually inactivate the virus, but risks to human health also have to be considered. This therefore raises the question – can we make disinfectants more effective at lower and safer working concentrations?
A note on norovirus models
The absence of an efficient culturing system for infectious human norovirus makes researching infection control strategies in the lab challenging. Typically, caliciviruses that can be propagated in cell culture, such as mouse norovirus (MNV) and feline calicivirus (FCV) have been used as models. Of these, MNV has the most efficient cell culture system and therefore has been used most extensively. To assess disinfectants, MNV is again the preferred choice, as it has been shown to be more environmentally stable than FCV. However, human norovirus (hNoV) itself is more robust than both surrogate viruses, and therefore better models are needed. Recent advances in 3D organoid models of human intestinal epithelial cells for culturing hNoV will allow us to enhance our understanding of the efficacy of disinfectants noroviruses.
A new disinfection strategy
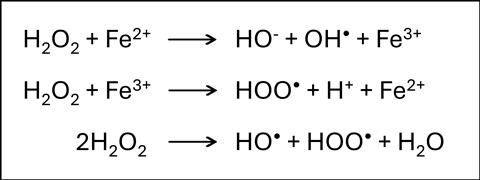
H2O2 acts as a disinfectant by producing reactive oxygen species (ROS) and free radicals that interact with and damage organic matter including proteins and genetic material. Inactivation of norovirus by H2O2 was found to be variable, depending on various factors including the concentration and time of treatment, presence of organic soiling, type of surface, and surrogate used. For instance, one study showed exposure to 35,000 ppm (3.5% w/v) activated H2O2 for ten minutes was required to inactivate murine norovirus (MNV), while feline calicivirus (FCV) could be inactivated with concentrations ten times lower. Another study indicated that fogging with 12.5% vapourised H2O2 for five minutes completely deactivated FCV, but did not remove hNoV.
Our work focuses on enhancing the disinfection efficacy of H2O2 against norovirus using an iron-based modified polyacrylonitrile (PAN) catalyst. Iron increases the production of ROS by reacting with H2O2 (Fenton’s Reaction). The PAN catalyst has previously been shown to enhance the action of H2O2 against various bacteria and spores, but this has not yet been extended to viruses. We hypothesised that in the presence of the PAN catalyst, lower concentrations of disinfectants such as H2O2, chlorine dioxide, and hypochlorous acid, all of which produce ROS, can be used to inactivate norovirus. Indeed, our preliminary results showed that the catalyst significantly increased the efficacy of dilute H2O2 (0.25% w/v) against murine norovirus (MNV-1), suggesting that lower concentrations can be used for efficient disinfection. Interestingly and unexpectedly, the PAN catalyst appears to decrease the disinfection activity of hypochlorous acid against MNV-1; further investigations to determine the reason for this are ongoing.
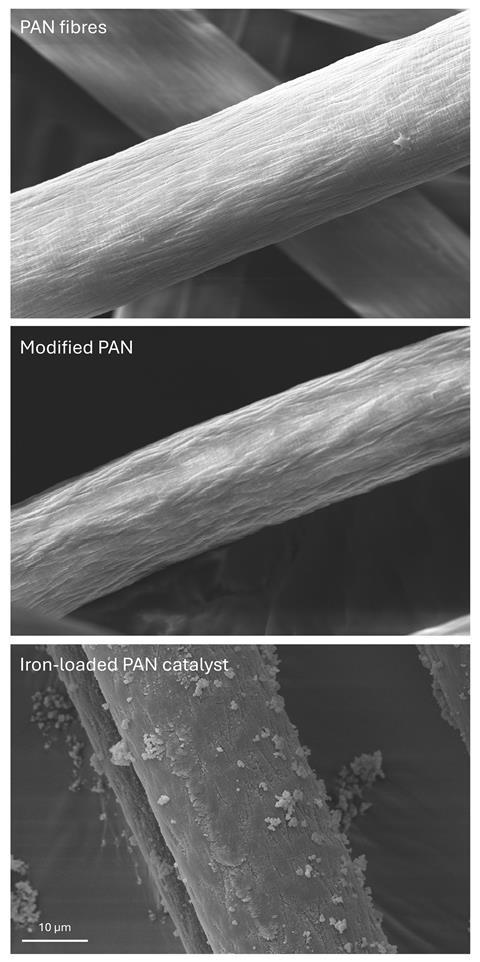
Future challenges
One important factor to consider is the development of resistance. Although disinfectants are not typically thought to generate resistance, bacterial susceptibility to disinfectants has been shown to significantly decrease with improper infection control practices. Resistance mechanisms of viruses are generally less well-studied, however, there are reports of disinfection generating resistance in poliovirus, enterovirus, rotavirus, and norovirus. Repeated exposure of murine norovirus to sub-optimal levels of free chlorine was found to lower its susceptibility to chlorine, with mutations in the minor capsid protein VP2 suggested to contribute to this resistance. Would lowering the concentration of disinfectants within our PAN catalyst system encourage the development of resistance mutations? Repeated exposure of MNV-1 to H2O2 has not yielded resistant viruses, however, we are still investigating treatments over longer time periods, a range of H2O2 concentrations, and other disinfectants.
Another aspect we are keen to explore is the sustainability of our disinfection system. We are currently investigating the reusability of the PAN catalyst. Preliminary work has indicated a low rate of iron-leaching from the catalyst, suggesting that the catalyst is likely to remain active over long periods of time. By ensuring the catalyst has high longevity and reusability, we aim to make our disinfection system a more sustainable and cost-effective solution for infection prevention and control.






No comments yet