Most of us are familiar with the phrase ‘gut feeling’, which according to the Cambridge dictionary is “a strong belief about someone or something that cannot completely be explained and does not have to be decided by reasoning.” This inexplicitly reveals the relationship between the gut and the brain.
What is the gut-brain axis?
The concept of a gut-brain connection is one that is familiar to all of us. For instance, most of us have experienced “gut-wrenching” when we feel nervous or under stress. Commonly, we feel “butterflies in the stomach” when we have an interview or something exhilarating happens. These nerve-racking feelings are associated with the release of neurotransmitters such as norepinephrine, the hormone that kicks in when our body is in a state of “fight-or-flight” response.
Simply put, the gut-brain axis is:
The bidirectional communication between the central and the enteric nervous systems, that connects cognitive performance to peripheral intestinal function.
A seminal study published in 2004 discovered that germ-free mice become more vulnerable to heightened stress responses and also are deficient in brain development. This trailblazing study uncovered that indigenous microorganisms are the key to regulating brain functions. Since the germ-free mice study first shed light on the significance of the commensal microbiota, other studies are suggesting an increasingly solid connection between the gut and the brain. Just as this study expanded our nascent understanding of the gut-brain axis, the health implications revolving around this physiological connection are becoming increasingly evident.
How does diet affect the gut-brain axis?
We know that what we eat affects our body and mind, but how do diets play an instrumental role in modulating our mood or health? As humans, we eat to sustain our lives like any other living organism, but how does the brain tell when we feel hungry or sated? This intricate feeding behaviour manifests in the sophisticated coordination between gut hormones and the brain. Have you ever noticed that we crave high-sugar or fatty food when we feel tense? This innate response has been hard-wired since our ancestors became hunter-gatherers, in order to help them survive. In modern days, we are saturated with abundant food choices with varying degrees of quality, and the burgeoning incidences of type 2 diabetes and metabolic syndromes are concomitant with the prevalence of ultra-processed food and Westernised diets. The craving for reward lies in the dopamine pathways of the reward circuitry in the brain, and a recent study published in Cell described how vagal gut-to-brain neurons are tied to sensory neurons in the upper gut, and to striatal dopamine release in the brain.
Whilst many mice studies have consolidated the idea of the gut-brain axis, we are still just uncovering the tip of the iceberg. You might not be unfamiliar with the term ‘psychobiotic’, which refers to live bacteria that render health-promoting benefits when administered in adequate amounts. These can be prescribed as adjuvants to treat psychiatric illnesses such as anxiety or stress therapeutically. A systematic review and meta-analysis found an association between disrupted gastrointestinal tracts and mental illness. For example, irritable bowel syndrome is associated with psychiatric disorders such as depression, anxiety, and schizophrenia.
Other unequivocal meta-analyses and cohort studies have concurrently demonstrated the efficacy of psychobiotic treatment and intervention. Broadly, these are probiotics, prebiotics, or diets rich in fermented food. Those dubbed as healthy diets are enriched in dietary fibre, cannot be directly ingested in the small intestine, fermented in the lower gastrointestinal tract, and feed beneficial microbes that are conducive to gut flora. One of the prevailing theories is that ingestible dietary fibre or resistant starch can be fermented in the colon and produce short-chain fatty acids, which modulate the neuro-immunoendocrine and control energy balance. What we feed to our gut microbes indirectly impacts our health.
Food digestion, human nutrient uptake and assimilation are complicated, each involving a series of enzymatic and biochemical reactions. Plus, we as individuals have different metabolisms and genetic makeups. Therefore we each have different responses to the same foods, which is combined with the fact that the quality and structure of food is not homogenous across the world. Foods are not a single nutrient in isolation such as you might find listed on grocery items, but a complex matrix of chemicals from various sources.
An ever-growing number of human studies deepens our knowledge about different dietary patterns and their impact on our health. We begin to appreciate dietary intervention and its pivotal role in preventing or exacerbating diseases or cancers – for example, the protective role of plant-enriched diets in many chronic diseases, or how ultra-processed food could aggravate metabolic syndromes. The issue of food allergy also draws our attention to the crucial interactions between food, host physiology, and the immune response. Addressing the underlying mechanisms of dietary impact on our bodies is, however, still costly and difficult to achieve.

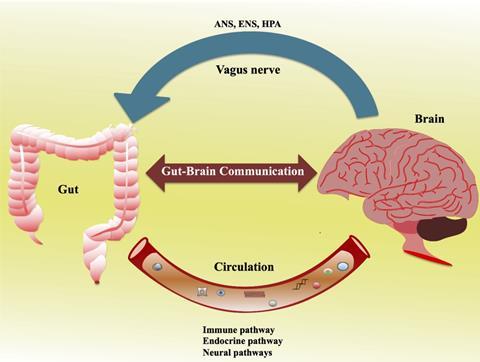
How does the gut communicate with the brain?
Animal studies have leveraged our mechanistic understanding of how diets affect the gut-brain axis. This two-way connection is modulated through two main routes including the neural (vagus nerve or spinal cord) and endocrine (hypothalamic-pituitary-adrenal axis). In addition, chemical, microbial-derived short-chain fatty acids (such as acetic acid, propionic acid, and butyric acid) and neurotransmitters released into the bloodstream may directly or indirectly signal to the brain to elicit behavioural changes, although the mechanism of action has not yet been fully elucidated. One of the plausible mechanisms is that under the insults of gut dysbiosis or infection, the intestinal barrier becomes permeable, known as a “leaky gut”, which increases the production of inflammatory cytokines and impacts the blood-brain barrier.
As well as the central nervous system (CNS) in the brain, the enteric nervous system (ENS) in the gastrointestinal tract has evolved independently, and it has a complex neural network and independently functions from those neurons in the CNS. The ENS is critical for the gut-brain axis and consists of neurons extending from the oesophagus to the anus, which governs the whole of the digestive process and motility. The gut can produce chemicals such as cytokines and neurotransmitters such as aminobutyric acid (GABA), dopamine, and glutamate. Of note, neurotransmitters are produced both in the brain (CNS) and gut (ENS), and more than 90% of serotonin (5-hydroxytryptamine) is produced in the gut. These neurotransmitters are essential for cognitive function, reward, mood control, learning, and memory in the CNS, whilst the role of endogenous serotonin released from the mucosa in the gut is still debatable. However, serotonin dysfunction is observed in a plethora of diseases which includes Crohn’s disease, ulcerative colitis and irritable bowel syndrome.
The vagus nerve is part of the parasympathetic nervous system and is the most widely distributed autonomic nerve that links the brain and other organs. It plays a critical role in human physiology. The vagus nerve consists of afferent fibers, efferent fibers, and vagal efferent fiber-endings located in the myenteric plexus, which directly interacts with gut microbiota and recognizes their secreted signals. For example, vagal afferent fibers are in direct contact with enteroendocrine cells (EECs), and they can sense the metabolites from intestinal commensal microbiota and produce gastrointestinal hormones or regulatory peptides such as ghrelin and glucagon-like peptide-1 (GLP-1). This process is essential to energy intake and food reward-motivated behaviours. The vagus nerve also releases different neurotransmitters or neuromodulators to control gut peristalsis and smooth muscle contraction.
The limitations of the gut-brain axis research
The interaction between the gut and brain is essential to maintain bodily homeostasis and health. The perturbation of gut microbiota has been linked to brain, mental, and cognitive health. The evidence is clear, but most preliminary studies are conducted using mice models and are hard to translate into humans due to the differences in anatomy between humans and mice. Even though some small interventional or observational studies are illuminating how certain bacterial genes are associated with eating behaviours and weight loss, this is not a cause-effect relationship. Longitudinal and interventional studies are necessary to determine how behavioural changes are associated with certain microbial species. Moreover, mechanistic studies of how gut microbiota impact brain function are still thin, which impedes the translation of identified microbes or microbe-derived metabolites into robust therapeutic targets.
Admittedly, the gut-brain axis is a fascinating yet equally challenging research topic in which to disentangle a causative relationship. It is not just limited to a distinct discipline or expertise in, for example, neuroscience or gut microbiology. We would also require a collaborative effort and multidisciplinary approaches to unravel the mechanism that underpins the connection, such as collaboration between endocrinology, immunology, bioinformatics, and epidemiology. We desperately need joint efforts to tackle outstanding issues such as food allergy and mental crises such as depression. Most importantly, we should renew our attitude towards nutrition and our understanding of diets, using holistic approaches that take into context the gut-brain interaction.








No comments yet