Fresh from placing in the top of inaugural The Future is Fungi awards, William Newell of Imperial College London opens up on his pioneering work which aims to use fungi for electromicrobial CO2 fixation.
I’m primarily interested in carbon fixation - the process in which carbon enters the biosphere is in my opinion the most important chemical process in the natural world.
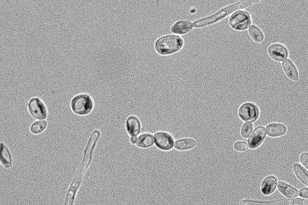
Initially, I was interested in fixing carbon directly from the atmosphere - like plants do. However, over the last few years, I have worked on teaching microbes how to assimilate single carbon (C1) compounds using new-to-nature biochemistry. These compounds can be made by electrically reducing CO2, allowing the microbe to act as an electronic carbon capture platform. Currently, I work on engineering this process in a fungus - Yarrowia lipolytica - for my doctoral thesis at the RLA lab at Imperial College London.
Massive shift
All climate goals set by society require a massive shift in how we make things, grow food and move around the world. Part of this gigantic effort will be figuring out how to convert carbon dioxide in the atmosphere into the chemicals we need to support our industrial civilisation. Biology has been absorbing carbon from the atmosphere for a billion years, but natural carbon fixation by photosynthetic organisms is space and energy inefficient, constricting the usefulness of biotechnology in tackling the climate crisis.
However, by using synthetic biology to create industrial microbes able to use electricity (in the form of hydrogen or reduced CO2 compounds like formate) as an energy source for CO2 fixation, we can create highly efficient ‘electrosynthetic’ biomanufacturing processes. This has been explored extensively in bacterial species, but these are delicate and so difficult to use at large scale.
Yeasts offer great industrial potential, but progress in feeding CO2 to yeasts has been limited. We are attempting to engineer this process in the fungus Y. lipolytica, a microbe which produces large amounts of potential petrochemical precursors.
Engineered strain
Last year, we engineered a strain of Y. lipolytica able to grow on the reduced CO2 compound formate. We then explored metabolic engineering strategies to improve growth on formate, allowing faster and denser growth. We also engineered Y. lipolytica to produce non-native bioproducts from formate.
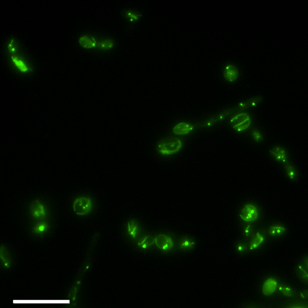
In general, this research has shown that single celled fungi are a rich - but unexplored - source of industrial microbes for CO2 conversion. Our research has added to a growing body of evidence that yeasts can host highly efficient carbon assimilation pathways, meaning that many unexplored fungi could be well-suited for growth on reduced CO2. We have also found that Y. lipolytica was able to synthesise energy-dense products, opening the door for biomanufacturing of numerous industrial chemicals from CO2.
Surprising finds
We’re taught that the biochemistry of microorganisms is set in stone, but the emergence of a novel kind of metabolic behaviour contradicts this orthodoxy. That was very surprising - the fact that you can rewrite a eukaryote’s metabolism using engineering approaches.
We’re currently working on sharing our findings with the world - but following this, work to industrialise our strain will commence and we will explore spin-out and patenting opportunities.
This strain is an exciting finding which shows the potential of yeasts as platforms for C1 biotechnology. However, substantial metabolic engineering, culture optimisation and process design will be required to fully industrialise this strain.
Chemicals from formate
Next, we want to explore the possibility industrial chemicals from formate, using synthetic biology to engineer new metabolic pathways for bioproduction. I’d also like to experiment with more exotic methods for C1 assimilation, potentially expanding the range of possible substrates to other CO2-derived compounds or using new-to-nature pathways to convert C1 to biomass.
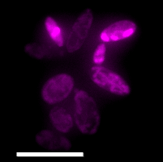
CO2 capture and recycling are vital for a sustainable future. C1 biotechnology could allow microbes to convert CO2 and electricity into almost all of the industrial compounds we use today, hugely accelerating the circularisation of the economy. With falling costs of green energy and advances in biotechnology such as these, we could live in a world built from CO2 by biology in the next decade.
To find out more about William’s work and the rest of the research happening at the RLA lab, visit https://www.rlalab.org/ and https://www.linkedin.com/in/macromicrobiologist/.
To find out more about The Future Is Fungi Award, click HERE.







No comments yet