Autoimmune diseases occur when the immune system mistakenly attacks the body’s own tissues. Psoriasis, an autoimmune inflammatory condition, affects about 125 million people worldwide. Common symptoms include red patches of skin with thick, silvery scales, dry and cracked skin that may bleed, itching, burning, soreness, and thickened or ridged nails.
Psoriasis can significantly impact people’s lives. Physically, it causes discomfort, pain, and itching, leading to sleep disturbances and difficulties in daily activities. The visible skin lesions can cause self-consciousness, social stigma, and emotional distress, contributing to anxiety, depression, and reduced quality of life. Managing psoriasis often requires long-term treatment and frequent medical visits, which can be time-consuming and costly.
Current treatments for psoriasis are limited. Typically, topical treatments with corticosteroids are used to temporarily relieve symptoms, but these can thin the skin, cause skin color changes, and lead to systemic side effects such as growth retardation.
”Instead of relying on synthetic drugs as a temporary solution, there is a strong need for more effective and sustainable solutions to chronic inflammation.”
Introducing the living hydrogel
The human body hosts trillions of microorganisms, roughly equal in number to human cells. These bacteria play active roles in modulating and maintaining the balance of our immune system. For example, Staphylococcus epidermidis, a common skin commensal bacterium, helps promote antibacterial protection, facilitate wound healing, and maintain tissue homeostasis through intricate interactions with the skin’s immune system.
Chronic skin conditions are strongly correlated with imbalances in skin microbiota, marked by reduced diversity of natural skin bacteria and over-proliferation of invasive strains like Staphylococcus aureus. This raises an important question:
“Can we address chronic skin inflammation by restoring the natural balance of resident bacteria?”
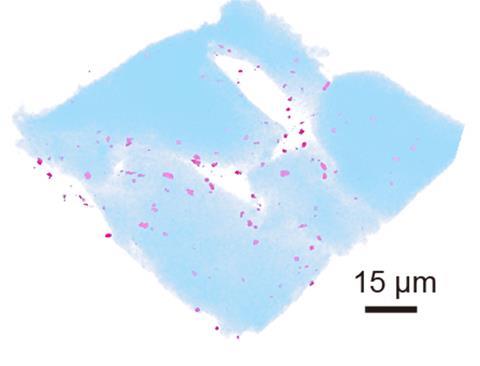
To explore this, we synthesized a ‘living hydrogel’ containing S. epidermidis. Specifically, we encapsulated S. epidermidis in a hydrogel matrix made of tapioca starch and gelatin, food-grade polymers sourced from nature. In this gel matrix, S. epidermidis could proliferate and maintain high viability. This living material can be freeze-stored for a month and rejuvenated without significant loss in bacterial activity, indicating scalability and transportability.
When we applied the living hydrogel to mice with imiquimod-induced psoriasis, psoriasiform features such as erythema, induration, and desquamation significantly decreased after four days of treatment. Bacterial secretes containing immunomodulatory substances were released from the living gel matrix, driving the therapeutic effects.
This discovery suggests that instead of using synthetic drugs with systemic side effects, we can use innate members of the skin microbiota and biogenic polymers to restore the natural balance of the skin and treat autoimmune diseases. The living hydrogel offered a sustainable, nature-inspired method of managing chronic inflammation.
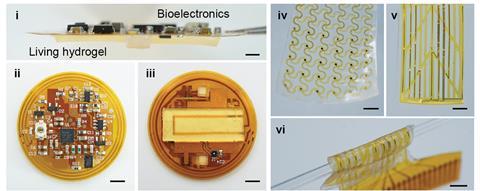
Bioelectronics
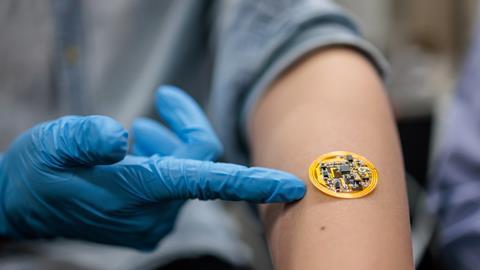
We didn’t stop at treating psoriasis; we also aimed to wirelessly monitor therapy progression and skin condition for real-time diagnostics. To achieve this, we integrated electronic components on top of the living material. The electronic component consisted of battery-free, wireless flexible printed circuit boards (FPCB) hosting commercially available sensors. The FPCB device possessed a near-field communication (NFC) transponder for energy harvesting and wireless data transfer. The sensors were used to monitor humidity, temperature, and impedance of psoriatic tissue. A reduction in impedance provided an indirect measure of reduced inflammation and skin thickening along the progression of psoriasis treatment.
In another design, the living hydrogel was integrated with a flexible electronics device for surface electrocardiogram (ECG) recording, measuring electrical signals from heart activity. In inflamed tissue with severe psoriatic features, the signal-to-noise ratio from ECG recordings was significantly lower than in normal tissue. Thus, electrophysiological recording could offer a quantitative measure of psoriasis severity.
Living bioelectronics: where does synergy arise?
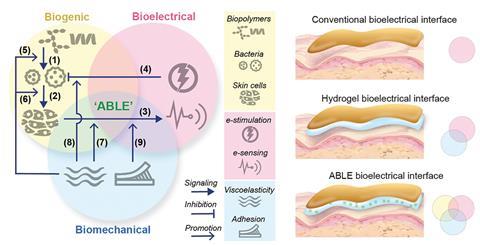
We have explained the role of living and electronic components in psoriasis management: the living hydrogel plays a therapeutic role, while electronics are used for monitoring and diagnostics. Altogether, we named this system Active Biointegrated Living Electronics (ABLE). In ABLE, how do the living and electrical components interact and synergize?
Firstly, electrical components resolve biosafety issues associated with living hydrogel. While S. epidermidis is relatively safe (biosafety level 1), using live bacteria in therapeutics still raises biosafety concerns. Most households lack biohazard disposal systems, and uncontrolled disposal could pose environmental risks. Additionally, S. epidermidis, though harmless in healthy individuals, could cause opportunistic infections in immunocompromised people.

To address this, we installed a safety trigger to disinfect the bacteria on demand. We integrated a pair of gold electrodes to apply an oxidizing current to the living hydrogel. Applying 30 minutes of oxidizing potential reduced S. epidermidis viability to nearly zero, with reactive oxygen species (ROS) responsible for this reduction. Overall, electrical components provided a non-genetic and controllable method to disinfect the living hydrogel.
Secondly, the mechanical properties of the living hydrogel enhance the efficiency of tissue monitoring and bacteria disinfection by the electronics component. Traditional bioelectronics lack an interlayer between tissue and electrical components, leading to gaps and poor signal acquisition, as well as unwanted inflammatory responses. In the ABLE system, the moisturized and adhesive living hydrogel, with tissue-like mechanical properties, bridges the gap between electronics and tissue. This improves the acquisition of electrophysiological signals used to monitor and diagnose psoriasis.
The future of living bioelectronics
How can we improve the functions of living bioelectronics to manage various autoimmune diseases beyond psoriasis?
Screening effectiveness of various commensal bacteria in autoimmune diseases
While our work focused on S. epidermidis for psoriasis treatment, this bacterium is also linked to other chronic skin conditions like dandruff and atopic dermatitis. It is crucial to evaluate the effectiveness of the bacteria in different chronic inflammation models. Given the thousands of bacterial species on the skin, many may play vital homeostatic roles. By developing in-vitro models, such as skin-on-a-chip, we can rapidly screen the immunomodulatory effects of various bacteria, creating a library of therapeutic strains for chronic skin diseases. A detailed understanding of bacterial-skin interactions will help in formulating living hydrogels with enhanced therapeutic effects.
Exploring synergistic interactions between electronics and living hydrogel
ABLE demonstrated that electronics can disinfect living hydrogel using an oxidizing current. Future advancements in living bioelectronics should explore additional modes of interaction between electronics and the living hydrogel. This will help in diversifying the outcomes of modulation beyond disinfection, enabling adaptive and personalized medication by training and modifying the living biointerface properties on-demand.
The following table presents some proposed interactions between bioelectronics and living hydrogel.
| Modes of interaction | Mechanism | Desired effects |
|---|---|---|
|
Oxidative |
The oxidizing potential applied by bioelectronics electrolyzes the living hydrogel, generating gases and reactive oxygen species (ROS). |
Toxic biocides, such as Cl₂, can disinfect bacteria in the hydrogel. In contrast, appropriate levels of reactive oxygen species (ROS) can promote bacterial growth and metabolism through eustress. |
|
Capacitive |
Some bacteria possess voltage-gated ion channels that open in response to capacitive electrical stimulation, leading to changes in the bacterial membrane potential. |
Changes in membrane potential can influence bacterial virulence, biofilm formation, and susceptibility to antibiotics. |
|
Electrogenic |
Some bacteria can exchange electrical charges with electrodes through electrogenic pathways. By applying the appropriate electrical potential, we can modulate the charge balance within the bacteria. |
The electrogenic system in bacteria can be connected to the physical properties of the living hydrogel, such as Young’s modulus. |
|
Photonic |
Micro LEDs in bioelectronics can modulate the living hydrogel. While some bacteria are naturally sensitive to light, the addition of photosensitizers, such as quantum dots, can convert photonic stimuli into chemical signals that affect bacterial metabolism. |
Retardation or promotion of bacterial metabolism and bioactivity |
In summary, we combined living bacterial components with electronics to create ‘living bioelectronics’ for synergistic psoriasis management. The living hydrogel offers therapeutic benefits, while the electronics enable monitoring and diagnostics of inflammation. The synergistic integration of the two components resolves biosafety concerns through an on-demand disinfection mechanism and enhances the efficiency of biological signal acquisition.







No comments yet