Bacterial vaginosis (BV) is a remarkably common condition among women of reproductive age, affecting as many as a third of all women at any given time. Yet it remains surprisingly elusive. Despite the word “vaginosis” bringing up images of overt symptoms such as discharge or odor, most cases of BV are actually asymptomatic, leaving both patients and clinicians unaware that anything is amiss. This subclinical presence, coupled with BV’s high prevalence and association with various diseases makes it a significant public health concern.
Introduction: bacterial vaginosis, common, but often asymptomatic
Why does this matter if so many individuals remain symptom-free? The short answer is that BV, even when silent, disrupts the normal balance of the cervicovaginal microbiome (CVM) and may tip the scales toward adverse gynecological outcomes, such as increasing the risk of acquiring sexually transmitted infections (STIs) like Chlamydia trachomatis. In other words, subclinical BV could set the stage for a variety of complications, from pelvic inflammatory disease (PID) to pregnancy-related issues.
Investigating BV in this subclinical form is inherently challenging (i.e. you can’t study what you don’t detect) and many affected individuals have no telltale signs prompting them to seek care. This also makes research involving BV challenging as many positive cases will be missed due to a lack of symptoms. Traditional diagnostic methods rely on Amsel’s criteria and the Nugent score, which, while valuable, are costly and time-consuming to perform. They are also only performed if a woman comes in with some complaint, such as vaginal discharge, indicating a possible diagnosis of BV. Given the difficulty of diagnosing BV without symptoms, there has been growing interest in molecular approaches that can sensitively and objectively identify this condition.
One such approach, which we have developed, is the molBV algorithm based on comparing samples from individuals with BV and positive by both Amsel criteria and Nugent score to those without symptoms and negative for both of these BV tests. From cervicovaginal samples converted into DNA and using molBV to classify the 16S rRNA data (which is like a genetic barcode present in all bacteria that allows us to identify different species), we were able to calculate a molecular BV score. The molBV score is similar to the clinical Nugent score, which is considered the gold standard for BV diagnosis, and uses a similar diagnostic scale of 1-10, where 7-10 is considered consistent with BV. Importantly, since it uses advanced technology that directly sequences a bacterial gene, it is objective and based on next-generation sequencing it can test thousands of samples simultaneously. This in turn enables a more nuanced and quantitative detection of BV and allows BV subtypes to be recognized as we have recently reported. In many ways, this “molecular diagnosis” shines a spotlight on the hidden forms of BV that might be fueling risk for STIs, including chlamydia. Significantly, not all forms of BV might be considered pathogenic although this requires more research.
In our recent study, published in Cell, we applied this algorithm to a longitudinal cohort of adolescent and young women to investigate the relationship between all forms of BV and incident Chlamydia trachomatis infection and subsequent reinfection which is a known problem. This article for The Microbiologist summarizes our findings and offers insights into the dynamic and pliable nature of the CVM, as well as the potential for targeted interventions as we unravel the complexities of bacterial vaginosis.
A hidden risk factor for STIs
BV is often subclinical
Bacterial vaginosis has traditionally been described by symptomatic markers and some clinical signs—thin, white discharge, raised vaginal pH, “clue cells” on a wet mount, and a fishy odor. Yet many women never experience the clinical symptoms or have hallmark signs. The subclinical presence of BV is problematic because it can go undiagnosed, persisting without intervention while silently elevating a patient’s susceptibility to infections like chlamydia.
For researchers, this lack of symptoms adds complexity to epidemiologic studies. Unless actively testing for BV regardless of symptoms, we risk underestimating the actual prevalence of BV and missing important risk factors that link BV to a host of adverse outcomes. At the population level, Black and Hispanic women experience higher chlamydia rates, and we’ve observed that BV also has a higher incidence in these populations. The reasons for these clinical differences are multifactorial, with socioeconomic and structural factors likely impacting both the microbiome and the risk of STIs.
The cervicovaginal microbiome (CVM) before, during, and after chlamydia infection
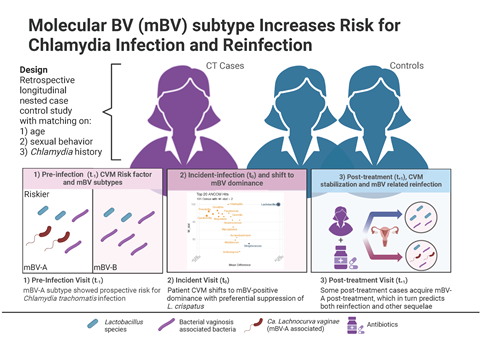
Using a prospective longitudinal research design, we studied the association of BV (and the CVM in general using an agnostic approach and multiple measures – molBV, Community State Types (CSTs), and Microbial Risk Score (MRS) with respect to the natural history of chlamydia infection. Specifically, we characterized the CVM at three distinct time points relative to an incident chlamydia infection:
- Pre-infection (t−1): About six months before diagnosis.
- Infection (t0): At the time chlamydia was diagnosed.
- Post-treatment (t+1): Around six months to a year after successful antibiotic treatment.
What we found was that even before the clinical diagnosis of chlamydia the women who went on to develop an infection had higher levels of certain anaerobic bacteria commonly associated with BV, including Candidatus Lachnocurva vaginae (formerly BVAB1) and Prevotella. Interestingly, this relationship wasn’t accounted for by differences in sexual behavior—in fact our study’s robust epidemiologic design matched cases and controls on background, and prior chlamydia history, which effectively eliminated the effect of sexual behaviors and isolated microbiome-driven risk factors.
Of further interest was the finding that post chlamydia treatment, one might expect a return to a completely normal, lactobacillus-dominant state, but many women remained in a transitional microbiome, teetering between healthy and dysbiotic states. An additional analysis revealed that this created a scenario ripe for reinfection, with the women who harbored the same risk-associated CVM identified in the pre-infection samples going on to become reinfected. Interestingly it appeared that in both the incident infection and subsequent re-infection BV did play a role, but not all BV. What we found was a certain sub-type of BV that was particularly risky for the acquisition of a chlamydia infection.
Introducing molBV subtypes: beyond a simple positive or negative
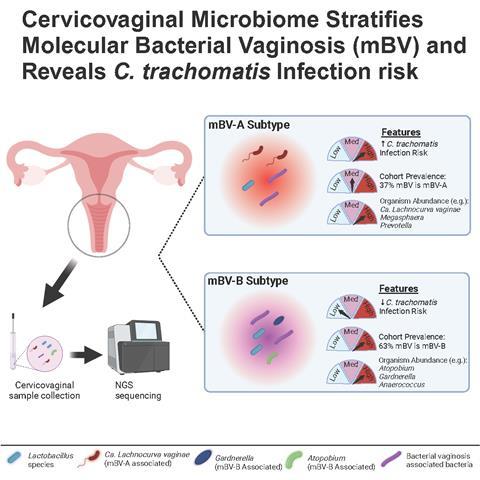
Traditional BV diagnostics categorize women as either “BV-positive” or “BV-negative.” Yet our data indicates that BV is not a single entity. There appear to be multiple forms, some of which may be more pathogenic than others. Here is where the molBV algorithm steps in. By leveraging 16S rRNA gene sequencing, molBV transforms the data into a single, continuous score (ranging from 0–10) that mimics the Nugent classification of BV and provides a result that has meaning to healthcare providers. Using this strategy, women fell into three categories:
- mBV-negative (scores 0–3): Typically high in Lactobacillus spp., especially L. crispatus.
- mBV-intermediate (scores 4–6): A transitional state with moderate levels of both protective and BV-associated bacteria.
- mBV-positive (scores 7–10): A polymicrobial state heavily enriched with anaerobes such as Gardnerella vaginalis, Prevotella, and Candidatus Lachnocurva vaginae.
In fact, what we found was that the mBV-positive state could be further stratified into two subtypes, one of which (mBV-A) conferred especially high risk for an incident chlamydia infection. This suggested that not all BV is equal; some subtypes may raise red flags, whereas others might simply reflect an alternative but less harmful microbial community. Black and Hispanic women.
Subclinical yet high-risk BV
Critically, using a molecular approach that targeted a specific bacterial gene combined with an automated platform in place of relying on subjective clinical symptoms also helped identify “silent” BV. Many of these women lacked a vaginal discharge or malodorous discharge yet harbored the microbiome features that promote acquisition of a chlamydial infection. These subtle changes would typically be missed using the standard approaches that directly depend on clinical manifestation of symptoms.
In short, molBV in combination with other features of the CVM exposes hidden layers of bacterial vaginosis, revealing a set of bacteria within an ecosystem that are risk factors for STIs. By quantifying and categorizing BV in this way, we not only explain how it can raise the risk for incident chlamydia but also shed light on why some women recover quickly post-treatment while others remain susceptible to reinfection.
When BV meets chlamydia: key findings
Prospective association of the CVM with incident chlamydia
By applying molBV to cervicovaginal samples collected before a chlamydia diagnosis, we discovered that high molBV scores (and the broad mBV-positive state) predicted a higher likelihood of future chlamydia infection within a six-month window. Additionally, by leveraging the species already identified using the 16S rRNA assay, we further observed that a specific form of mBV (mBV-A) - characterized by Candidatus Lachnocurva vaginae - seems especially important in driving acquisition of a chlamydia infection. These results held even after the rigorous matching of participants on behavioral risk factors, suggesting the CVM itself is playing a pivotal role in susceptibility and not other variables, such as sexual behaviors.
Reinfection and long-term sequelae
The story does not end after a single infection. Our follow-up samples collected after antibiotic treatment showed that, while chlamydia was eradicated, the CVM often did not return to a stable, lactobacillus-rich state. Instead, women with BV-like microbiomes pre- or post-treatment were far more prone to reinfection, reinforcing that treating the pathogen alone is not enough. This is consistent with the well-known observation that women with a treated chlamydia infection are more susceptible to repeat infections. We also observed potential links between post-treatment microbiomes and other long-term reproductive complications, including PID and miscarriage, although these findings were exploratory. The interesting fact was that it appeared the CVM was the risk factor and not chlamydia, although further studies are needed. This work underscores an important point, the cervicovaginal environment has broad consequences for women’s reproductive health and deserves closer monitoring and consideration for targeted interventions.
Mapping the ever-changing CVM: a microbiome in flux
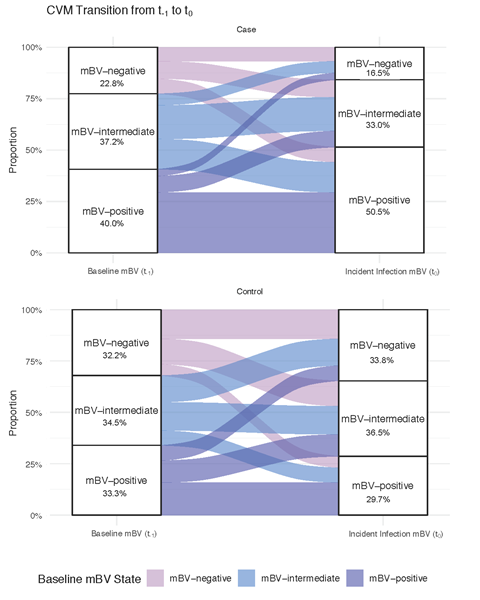
A unique aspect of our study was the multi-visit sampling for both cases and controls. Investigating samples over time revealed that that the CVM is constantly evolving in many but not all participants in this study (see alluvial plot above). This is true even in individuals who are classified as controls within the cohort. These plots trace how a participant’s microbiome composition shifts over time, resembling streams of color that split and merge, highlighting transitions between mBV negative (e.g., Lactobacillus-dominant states) and BV-like states. Why is this important? If the CVM is pliable under unknown conditions, then there should be an opportunity for intervention upon further knowledge of what drives the changes. Even if a woman’s microbiome transitions briefly into a dysbiotic, high-risk BV state, it could be a critical period for STI acquisition, such as Chlamydia trachomatis. By understanding and potentially steering these transitions, we have an opening to reduce infection risk and possibly other outcomes associated with BV.
Bridging STI Outcomes amongst different populations
The disproportionate burden of chlamydia among Black and Hispanic women, who face infection rates five times higher than their white counterparts, reflects a stark intersection of biological and social differences. BV also tracks differently in these same groups, albeit not always to the same degree (i.e. black and Hispanic women have BV prevalence ranging 30-40% compared to 20-30% in other demographic groups). Our cohort in New York City included over 70% Black and Hispanic adolescents and young adults. This population is often underrepresented in microbiome and STI research, which is particularly problematic given the prevalence of chlamydia in this group. By focusing our work on individuals most heavily impacted by chlamydia, we ensured more informative data for the population most affected by this infection. This in turn is crucial for designing interventions that work in real-world settings. Understanding the pathogenesis of a disease and/or infection is paramount to developing interventions and how to use them for the greatest benefit.
Conclusion
Taken together, our findings urge a rethinking of chlamydia prevention and, by extension, many other STIs influenced by a dysbiotic or non-native cervicovaginal microbiome. BV (particularly subclinical BV) should not be ignored in reproductive health research. The dynamic nature of the CVM highlights both the complexity and the potential for timely interventions. With more research, we may be able to incorporate molBV or similar tests into routine gynecological check-ups, especially for high-risk populations. Identifying women with high-risk CVMs can allow for prophylactic treatment using prebiotics, probiotics, microbiome restoratives, or selective antibiotics aimed at shifting the CVM away from high-risk states (e.g., mBV-A) (although more research is needed to determine the effectiveness of such therapies).
Subclinical BV is more than a silent inconvenience; it’s an active, under-recognized factor fueling the spread of STIs like chlamydia and possibly setting the stage for other reproductive health complications. Armed with molecular diagnostics of BV and a deeper understanding of how dynamic the CVM truly is, we are poised to make significant strides in personalized prevention strategies, bridging the gap in STI outcomes across at-risk communities, and ultimately enhancing women’s reproductive health on a global scale.
Suggested Further Reading and Resources
Chlamydia and CVM: https://www.cell.com/cell/fulltext/S0092-8674(24)01424-7
molBV development and testing: https://www.nature.com/articles/s41467-021-27628-3
vaginal microbiome of reproductive age women: https://www.pnas.org/doi/full/10.1073/pnas.1002611107








No comments yet