In July of 1976, 2000 members of the U.S. military veteran’s organisation, the American Legion, attended their 58th annual three-day conference at the Belle-Vue-Strand hotel in Pennsylvania. In the following weeks, 182 severe cases of community-acquired atypical pneumonia were diagnosed, with 29 deaths. While the hotel was identified as the common point of origin, the true extent of the outbreak has been the subject of much debate.
In January 1977, the causative agent of the disease was finally classified as the new species Legionella pneumophila. Other members of the genus Legionella were soon discovered and shown to be ubiquitous in freshwater sources. Members of this common genus are all around us in water but are particularly problematic in warm water, typically found in building services, evaporative cooling systems, indeed anywhere where there is warm water.
Unlike many waterborne pathogens, Legionella can be considered a normal inhabitant of water systems. In intensively treated potable supplies, the presence of faecal indicators is a relative rarity, but Legionella is one of the more commonly isolated organisms. Its presence can be expected in many samples, but clearly, the degree of exposure will relate to an increased risk of clinical disease. Given the ubiquity of environmental Legionella spp., a risk-based approach has been employed by most high-income countries, where the bacterial load quantified from a water sample is far more meaningful than the simple presence or absence of the organisms. Unfortunately, the current testing methodology, while strictly standardised, may not provide an accurate indicator of the numbers present. In my own laboratory, we anticipate that up to 75% of Legionella spp. are lost during processing, and feel the current methods provide a significant under-estimation of the population due to these significant losses during analysis, as well as a complex life cycle involving viable but nonculturable stages.
Legionella bacteria are particularly fastidious, being particularly sensitive to agar-related toxins and having an absolute requirement for particular amino acid supplements for laboratory growth. The processing of water samples involves several steps to ensure the bacteria are properly cultured and can be studied effectively. There is an international standard for Legionella culture (ISO11731:2017), setting out the required parameters for optimal bacterial culture. Although this is a recent standard, it should be noted that culture methods for Legionella spp. have not advanced since buffering the medium with N–2-aminoethanesulfonic acid (ACES) was added to the agar formulation of yeast, activated charcoal, ferric pyrophosphate and cysteine hydrochloride since it was instituted by Pasculle et al. in 1980, and confirmed by Edelstein in 1981.
While relatively simple in composition, this medium will also grow many other organisms present in environmental water samples and a variety of selective antibiotic supplements have been used in an attempt to suppress non-Legionella competing species, but with only limited success. The slow growth of cultured Legionella (usually requiring 3-10 days to become visible), generally means that the competing flora often overgrow and obscure the detection of Legionella in those samples. This is particularly true of samples with a high microbial load (e.g. samples from cooling systems, open to the atmosphere – the very systems we might expect to be also harbouring excess growth of Legionella spp.
In addition to ISO11731 there is a published British Standards Code of Practice for Legionella sampling (BS7592:2022) and both documents feature prominently in UK and European regulatory guidance for Legionella control (See Table of relevant guidance and standards). ISO11731 sets out a variety of different pathways for samples, dependent upon sample type and expected bacterial load. However, most water testing labs follow the process described below:
1. Filtration
Samples are aseptically filtered through a 0.2µM filter, and the filter is removed to a small volume (typically 10-25ml) of low osmotic potential saline solution. The suspension is shaken or ultra-sonicated to remove the bacteria from the membrane, leaving a suspension of bacteria in the saline. In our laboratory, internal QC data suggests this is a large source of loss of viable cells during processing and may lead to a significant underestimation of Legionella numbers in the sample. Portions of the suspension may be treated with heat (50oC/30min) or acid buffer solution as a selective process.
2. Culture Media
Buffered Charcoal Yeast Extract (BCYE) Agar base media: the primary medium used for growing Legionella is BCYE agar. This medium contains charcoal to absorb toxic compounds, yeast extract which provides necessary trace nutrients, ACES buffering agent to maintain pH stability, L-cysteine and iron salts essential for the growth of Legionella spp.
The base media is supplemented with a variety of different selective cocktails, e.g. Glycine-Vancomycin-Polymyxin B-cycloheximide Agar (GVPC) or Polymyxin B-sodium cefazolin -Pimaricin (known as BCYE +Antibiotics). While GVPC is considered highly selective, it is also known to suppress some Legionella growth, thus representing another source of underestimation of quantification for the sample.
3. Inoculation and incubation
A small volume (typically 0.1 to 0.5ml) of the sample is spread onto the selective agar plates. Dilution plating can be performed if numbers of Legionella spp. are expected to be high. Samples of clinical origin (e.g. lung aspirates) obviously don’t require filtration, but follow the rest of the procedure from here, although they often favour BMPA (polymixin B-cefamandole-anisomycin) as the selective cocktail.
4. Incubation
Plates are incubated at 35-37°C. A humidified atmosphere is maintained, often achieved by placing the plates in a container with a water source to prevent drying or by wrapping the plates in plastic bags to create local humidity over the extended incubation required. Plates are typically incubated for 7-10 days, as Legionella has a relatively slow growth rate compared to other bacteria.
5. Identification
Legionella colonies on BCYE agar usually appear as greyish-white, slightly convex, and have a “ground-glass” appearance. Suspect colonies are usually subcultured onto BCYE base medium with cysteine and iron, and onto the BCYE base medium without cysteine. Growth on the former and not the latter is taken as confirmation as Legionella spp. as these bacteria have an absolute requirement for cysteine supplementation. Further identification can be done using latex serology, MALDI-TOF analysis, or molecular techniques.
A new media?
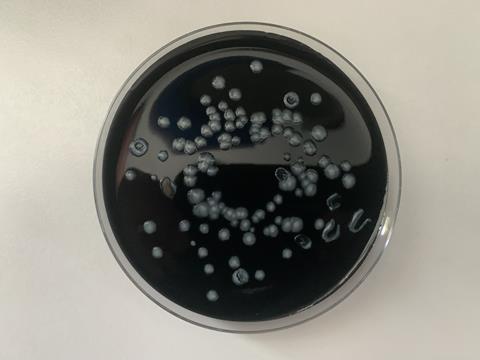
The charcoal agar is opaque and jet black, making automatic plate reading difficult because colonies cannot be observed through the bottom of the plate, and condensation on the lid of the plate (in the humidified chamber) requires lid removal to observe colonies. Other waterborne pathogen detection has benefitted from the development of chromogenic agar using enzyme-specific substrates that give putative species identification based on colony colour, but Legionella detection still requires an extensively trained technician and many hours of manual plate reading. The test is slow compared to most other waterborne pathogen tests, and the 10-day incubation period leads to frustrating delays for end-users. Each sample requires up to 3 plates to be inoculated for heat-treated and acid buffer portions, tripling the media cost. Added to this are the many problems of Legionella being obscured by overgrowth of competing waterborne flora (see photographs).
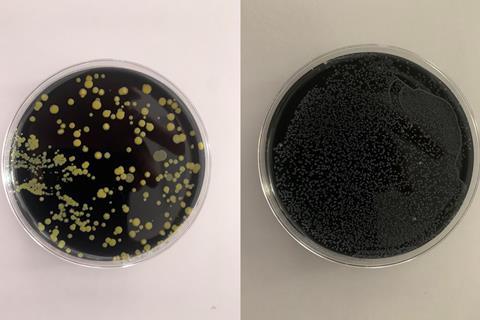
The first advance in solid Legionella culture methods since 1980 arrived in 2021 with the publication of a transparent, charcoal-free medium initially designed for antimicrobial susceptibility testing of Legionella pneumophila, LASARUS (for Legionella Antimicrobial Sensitivity and Resistance Universal Screening). This transparent, straw-coloured medium would be ideal for adaptation to a chromogenic environmental screening tool for putative Legionella spp., making colonies easily identifiable through the bottom of the plate. With the addition of the standard selective cocktails, it would also lend itself easily to high throughput automation for water sample testing. The addition of vancomycin makes LASARUS selective against Gram-positive organisms, particularly useful for screening clinical respiratory samples where these are common, while a carefully defined addition of colistin appears to allow Legionella growth while suppressing a majority of Gram-negative organisms common to environmental samples. Preliminary evidence also shows that Legionella colonies are larger (and therefore appear earlier) on LASARUS compared to BCYE, which is also very encouraging for faster culture quantification in the future.
Alternative technologies
Some laboratories have reported success using direct fluorescent antibody testing for Legionella, but the sensitivity is poor, and the specificity is open to question due to subjective factors. More recently, a great deal of work has been done to validate the use of Quantitative Polymerase Chain Reaction (qPCR) methodologies for Legionella. These tests require careful interpretation and are at present used more widely in outbreak situations as an initial screen to quickly identify positive sample points for further culture analysis. The high Negative Predictive Value for qPCR helps rule out potential sources in rapidly moving outbreak situations. A recent update to the current technical guidance (HSG274) also recommends qPCR as a complementary test to culture, but culture remains the gold standard methodology.
Testing and Regulation
Accurate testing is an important prerequisite for effective regulation. Attempts by government and non-governmental bodies to legislate and provide guidance have usually followed significant outbreaks of disease but have usually been successful in reducing future outbreaks. The United Kingdom has a long history of legislative requirements and the provision of technical guidance to assist end-users. This is led by the UK’s Health and Safety Executive (HSE), who recently published an update to the guidance for open evaporative cooling systems (HSG274, 2nd edition). Parts two and three of the same series provide guidance on the control of Legionella in hot and cold water services and other risk systems. Separate guidance covers Legionella and other infectious agents in spa pools (HSG282), and the regulatory framework is completed by an Approved Code of Practice. Failure to control risk in any workplace setting is potentially a legal breach and can have significant legal consequences.
When setting the target values, regulators take into account the uncertainty factors, and the UK has set very low limits for acceptable counts. Those limits are less than 100cfu/L for non-healthcare premises whilst in healthcare premises, any isolate is considered potentially significant. Some European countries have followed the UK lead, whilst others have adopted higher values for Legionella spp. counts. Generally, results of ‘None isolated’ are desired by all, but what does that result mean in a test prone to overgrowth and underestimation? ‘None isolated’ certainly does not mean ‘not present’ and control measures such as temperature control, biocides dosage, and overall cleanliness must be maintained, whatever the test result. Testing has a role, but the desire for improved testing methods and tools remains.




No comments yet