The ocean, constituting nearly three-quarters of the earth’s surface, plays an essential role in sustaining an integral part of life on the planet, shaping ecosystems, and influencing global processes. Oil spills have long posed a significant threat, with profound impacts on marine ecosystems.
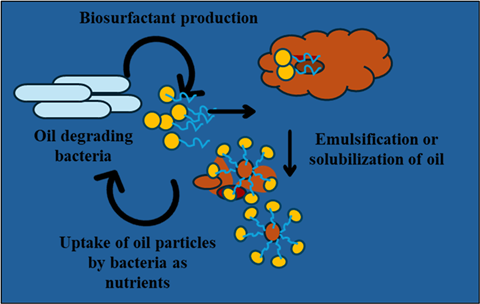
Some of the major causes resulting in an oil spill accident include transportation of oil, natural disasters (like explosions and hurricanes), leakages in pipelines, technical failures, or human error. The consequences of oil spills cascade through the food chain to affect birds, humans, and other species because of the domino effect. To count on a few, oil spills across large areas of seawater disturb oxygen circulation for marine organisms, cause hypothermia in birds, adversely affect navigation routes, and hinder anthropogenic actions like fisheries and tourism. In this regard, biological treatments appear to be a promising method and offer a sustainable solution. Typically, microbial bioremediation facilitates the simultaneous biosynthesis of surface active molecules (biosurfactants). Biosurfactants are eco-friendly with a low critical micelle concentration value, enabling diverse environmental applications.

Addressing the oil spill crisis: the power of bioremediation solutions
While physical, chemical, and thermal remediation methods offer potential solutions, they are often incomplete, costly, and sometimes introduce additional environmental challenges. Thus, an urgent need for an effective and environment-friendly solution drives us toward exploring biological treatment. The biological treatment process deals with the utilization of microbial populations possessing an inherent capacity to eat and consequently degrade oil. It is a bioremediation practice facilitated by the simultaneous production of biosurfactants by the oil-consuming microbes (as their food). Biosurfactants are amphiphilic molecules (possessing a hydrophobic tail and a hydrophilic head group) and can be classified into different categories depending on their chemical and structural organization. They are structurally categorized into high-molecular-weight and low-molecular-weight compounds. The low-molecular-weight biosurfactants usually include glycolipids, phospholipids, and lipopeptides, whereas the high-molecular-weight category includes lipopolysaccharides, proteins, and polymeric substances. These molecules carry functional groups in their head region that can bear positive or negative charges or can be neutral or zwitterionic (carrying both positive and negative charges). Fatty acids contain a hydrophobic tail.
The most widely studied biosurfactants are rhamnolipids and surfactin, which have garnered significant attention for their unique properties. Rhamnolipids, belonging to the glycolipid class, are predominantly produced by Pseudomonas species. In contrast, surfactin, classified as lipopeptides, is primarily synthesized by Bacillus species. These biosurfactants are of immense interest due to their versatile applications and remarkable surface-active properties. These wonder molecules have been known to promote soil and water health (by cleansing the oil-contaminated sites).
Unveiling the remarkable properties of biosurfactants: nature’s magic molecules
The characteristic properties that make these molecules a fascinating entity are their ability to alter the wettability/ surface energy, promote dispersion and emulsification between two immiscible liquids, and allow solubilization and mobilization of two dissimilar liquids.
These surface-active molecules also act by enhancing the cell-surface hydrophobicity of microbial cells by embedding their hydrophobic tails within the microbial cell membrane while exposing their hydrophilic heads to the external environment. The amphiphilic nature enables the biosurfactant-producing bacteria to more effectively adhere to and decompose hydrophobic substances, such as oils, being significantly useful in applications like bioremediation and improved oil recovery. Additionally, biodegradability, cytocompatibility, and their inherent stability offer an edge over their chemical counterparts.
Biosurfactants (depending on their concentration) act on recalcitrant contaminants (like crude oil) through mobilization, solubilization, or emulsification. Furthermore, their stable nature allows these tiny molecules to retain their functional properties throughout a wide spectrum of environmental conditions, including high pH, temperature, pressure, and salinity. The rigorous conditions under which these biosurfactant molecules have been evaluated and demonstrated stability encompass pH levels (2-12), temperature ranges (-20 °C to 100 °C), and salinity (3-21% (w/v) of NaCl). The remarkable stability of biosurfactants under extreme conditions makes them ideal for a wide range of applications. In microbial-enhanced oil recovery (MEOR), biosurfactants can withstand high temperatures, pH, and pressure, thriving in challenging environments. Additionally, their effectiveness in treating oil-contaminated sites, such as seawater bodies (marine ecosystems) with elevated salinity, highlights their potential for environmental remediation under harsh conditions.

Impact of biosurfactant concentration on enhancing bioremediation efficiency
The biosurfactant molecules usually allow the mixing of oil and water, facilitating the degradation of oil (hydrocarbons) by making it more bioavailable to microbial cells. The tendency of biosurfactants to decrease the surface tension of liquids is concentration-dependent, i.e., the higher the biosurfactant concentration, the greater the reduction in the surface tension. However, after a definite concentration, there is no further decrease in the surface tension value; this concentration is termed the critical micelle concentration (CMC). Consequently, a lower CMC value indicates greater effectiveness of biosurfactant molecules. The reported range of CMC values for surfactin is approximately 10 to 500 ppm. When these biosurfactant molecules are used at concentrations below their CMC, mobilization of oil contaminants takes place, which aids in reducing the capillary forces (between oil and soil), thereby decreasing the IFT and elevating the mobility of oil for bacterial remediation. If the concentration of biosurfactants is exceeded beyond the CMC, micelle formation takes place, encapsulating the oil droplets within their cores, causing their solubilizing.
Previous studies reported that the maximum crude oil degradation takes place at 0.5 CMC of biosurfactant. Hence, the CMC value of biosurfactants also plays a critical role in deciding their mode of action and is accordingly chosen for different applications. The biosurfactant molecules at a specific concentration enhance the surface properties of liquids by finely tuning the interaction between oil and water and establishing a feedback mechanism. An increased number of oil droplets, when dispersed into smaller particles, enhances the availability of nutrients for bacterial survival, resulting in their elevated proliferation. This, in turn, leads to an augmented production of biosurfactants, as they are typically regarded as growth-associated molecules. A comparable investigation conducted by a research team revealed that the degradation rate of crude oil was 0.004 ± 0.001 g/L/h, facilitated by the biosurfactant-producing bacterium Agrobacterium fabrum SLAJ731.
In order to further enhance the rate of biodegradation, the biological method using biosurfactant-producing microbes can be integrated with a physical biosorption process. The idea is to adhere microbial population over adsorbent materials (like sugarcane bagasse, cotton fibers, sawdust, and coir fibers) that have the propensity to adsorb oil, hence maximizing oil accessibility to the microbes. The key factors for sorbent selection encompass elevated adsorption capacity, selectivity, stability, reusability, and biodegradability. A study revealed that the adsorption rate for hydrophobic octyl-surface modified sugarcane bagasse was 2.5 times greater than that of untreated bagasse. The rate of microbial oil biodegradation increased 17-fold in the combined process. This integrated method synergistically expedited the overall cleanup of crude oil contamination.
Bioprocess economy of biosurfactant production
One of the biggest challenges of producing biosurfactants lies in their overall production cost. The selection of potential microbes and a suitable carbon source (that accounts for about 50% of the total cost) is crucial for adequate biosurfactant production. The isolation, screening, and identification of indigenous microbes from different target sites like oil reservoirs, marine/seawater (or coastal areas), and oil-contaminated sites (soil and water), oil-based fermented food products can be a major step in augmenting biosurfactant production yield. To further cut down on the production cost, cheap nutrient sources like molasses, waste cooking oil, corn-steep liquor, glycerol, banana pseudo stem, tea waste, and bamboo shoot processing waste can be some promising alternatives. While some of these have been previously studied, others represent potential avenues for further research.
A few potential bacterial strains that have been isolated include Bacillus subtilis RSL2 (from contaminated sludge), B. subtilis MG495086 (from formation water), B. subtilis SMP-2 (from a mustard-oil-based fermented food product), Agrobacterium fabrum SLAJ731 (from a core sample of an oil refinery), and B. subtilis IS5 (from seawater). Downstream processing includes separation via solvent extraction followed by vacuum evaporation and further purification. Moreover, the processes associated with downstream processing for the extraction of pure biosurfactants entail significant expenses. Consequently, research should primarily focus on the use and effectiveness of crude biosurfactants to minimize the cost-intensive steps of the separation and purification steps and provide a sustainable alternative.
Additional properties and applications of biosurfactants
Apart from altering the interfacial properties, biosurfactant molecules have also exhibited antimicrobial and anticancer activities. These features are harnessed for biomedical applications like antibiofilm formation, wound healing, and enhancements in skin and oral cavity care. Biosurfactants have demonstrated potent antimicrobial properties against a wide range of pathogenic bacterial and fungal species. Surfactin has been shown to exhibit potent antibacterial activities against a broad spectrum group of pathogenic strains, with a minimum inhibitory concentration (MIC) ranging from 30 to 80 μg/mL and a MIC against pathogenic fungal strains ranging from 5 to 100 μg/mL.
These molecules are expected to disrupt the cell walls of the bacteria, bind with bacterial membrane protein, causing their lysis or release reactive oxygen species (ROS), culminating in bacterial rupture and cell death. In a study, a nanoemulsion formulated with surfactin, doxorubicin (DOX), and essential oil exhibited a synergistic impact, enhancing both antibacterial and anticancer effects. Further, the charge-bearing surface active molecules can generate pores in the cell membrane of living systems, enabling their application as microemulsions in drug delivery systems (MDDS). Surfactants form an integral part of the modern commercial laundry and cosmetic industry.

Nonetheless, with the growing awareness of environmental risks linked with chemical surfactants, there is an imperative necessity to shift to eco-friendly substitutes—biosurfactants. These natural surfactant molecules are gradually being utilized in personal care products, like facewashes, soaps, shampoos, toothpaste, perfumes, and other skincare items. This is due to their proficient emulsion-forming capabilities while interacting with oils, as well as their notable antibacterial, anti-adhesive, and antifungal properties.
Certain biosurfactants are recognized for their ability to maintain the equilibrium of skin microbiota because of their antimicrobial potential. Moreover, the cleansing enzymes incorporated in detergent formulations, such as lipases, proteases, and amylases, exhibit efficacy; nevertheless, due to their proteinaceous composition, they are susceptible to deactivation under extreme environmental conditions, including elevated pH and temperature. Biosurfactants, by contrast, exhibit stability with minimal loss even under harsh conditions, rendering them more advantageous. Further, their application in MEOR is highly promising, as they tend to increase the capillary number (defined as the ratio of viscous force to capillary force), thereby improving oil recovery.
Towards an economic and sustainable future
It is an established fact that the wonder molecules (biosurfactants) play a pivotal role in diverse fields beyond their capacity to improve soil and water quality by remediating oil-contaminated sites. To boost biosurfactant production and, in turn, improve oil remediation efficiency, genetic modifications of bacterial strains can be performed by targeting genes responsible for biosurfactant-synthesizing enzymes. Additionally, designing a potential consortium comprising different bacterial strains can provide a more effective approach to dealing with oil contamination. Given that oil is a complex mixture of both aliphatic and aromatic hydrocarbons, there should be a wise and judicious selection of bacterial strains to escalate the degradation process. Further, it becomes essential to devise a strategy to efficiently recover the biosurfactants (synthesized by microbial strains) during the remediation process. This strategy would pave for a new gateway in developing the concept of circular bioeconomy. The recovered biosurfactant can be subsequently repurposed for different industrial applications, further enhancing its value and sustainability.
Further reading:
https://pubs.acs.org/doi/10.1021/acs.langmuir.4c04093
https://pubs.acs.org/doi/10.1021/acs.iecr.1c00974
https://doi.org/10.1080/25740881.2020.1784222
https://doi.org/10.1016/j.biortech.2020.123261
https://doi.org/10.1016/j.bcab.2019.101322







No comments yet