Imagine if we could explore the entire molecular universe in our bodies, interpreting the intricate chemical changes related to health and disease at its most fundamental level. This is the promise of metabolomics, a rapidly evolving field that’s revolutionizing our understanding of biology and medicine. But what exactly is metabolomics, and why is it generating such excitement in the scientific community?
At its core, metabolomics is the comprehensive study of metabolites – small molecules that are the intermediates and products of metabolism. These include familiar compounds like amino acids, lipids, and carbohydrates, as well as countless others, many of which are not yet known or described. Unlike other “-omics” sciences that focus on genes or proteins, metabolomics provides a direct snapshot of cellular activity at a given moment, offering unique insights into biological processes.
While genes remain relatively stable throughout life and provide a blueprint of potential cellular activities, metabolites are in constant flux, changing rapidly in response to environmental factors, diet, and physiological states. This dynamic nature of metabolites allows metabolomics to provide a real-time readout of cellular processes, capturing the immediate biochemical state of an organism, including what’s happening in our bodies. This makes it an invaluable tool for understanding health, disease, and how our bodies interact with the environment.
Techniques
So how do scientists actually perform metabolomics studies? The two primary analytical techniques used are mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. Mass spectrometry, often considered the gold standard, is like a super-sensitive scale for molecules. It can identify and quantify metabolites based on their mass-to-charge ratio, often detecting compounds at incredibly low concentrations – parts per trillion in some cases. NMR spectroscopy, on the other hand, provides detailed structural information about metabolites, once we are able to isolate them in sufficiently large amounts or produce them synthetically.
These sophisticated tools allow researchers to detect and quantify a wide range of metabolites with high precision, but the approach to exploring them can vary. Metabolomics studies generally fall into three categories: targeted, untargeted, and semi-targeted.
Targeted metabolomics is like going to a store with a specific shopping list. Scientists know exactly which metabolites they’re looking for and focus their analysis on those specific compounds. This approach is particularly useful for hypothesis-driven research or when studying well-characterized metabolic pathways.
Untargeted metabolomics, in contrast, is more like exploring the entire store without a list. Scientists attempt to detect and measure all metabolites present in a sample, without any preconceived notions about what they might find. This approach is excellent for discovery-based research and can lead to the identification of novel metabolites or unexpected changes in metabolic pathways.
Semi-targeted metabolomics, a hybrid approach, combines elements of both targeted and untargeted methods. It’s like going to a store with a shopping list, but when you reach the shelf for an item on your list, you also take the time to examine all the nearby products. You might find something unexpected that’s relevant to your needs. This approach offers a balance between focused investigation and discovery. It allows researchers to quantify specific metabolites of interest while remaining open to detecting related or unexpected compounds. This makes semi-targeted metabolomics particularly useful for studies requiring both accurate quantification of known metabolites and the potential to uncover new, relevant biomarkers or metabolic pathways.
Applications
The applications of metabolomics are as diverse as they are exciting. In medicine, it’s revolutionizing disease diagnosis and treatment. Researchers use metabolomics to identify biomarkers for early detection of diseases like cancer, diabetes, and cardiovascular disorders. This approach allows for more personalized treatment strategies, as metabolic profiles can indicate how an individual might respond to specific medications.
In drug development, metabolomics helps scientists understand the effects of new compounds on cellular metabolism, potentially accelerating the discovery of more effective and safer drugs. Nutritionists use it to assess the impact of different diets on human health, while environmental scientists employ metabolomics to monitor ecosystem health and detect pollutants in water and soil.

As the field of metabolomics continues to advance, we’re seeing a convergence of technological developments that are making this powerful analytical approach more accessible. This accessibility is opening up new possibilities for applying metabolomics in practical, real-world scenarios, including personal health diagnostics. One area where this is particularly relevant is in the monitoring of gut health, which has emerged as a critical factor in overall well-being.
Our understanding of the gut microbiome and its profound impact on health has rapidly evolved in recent years. The trillions of microorganisms residing in our digestive tract play crucial roles in various aspects of our health, from digestion and nutrient absorption to immune function and even mental health. The composition and activity of this microbial community are heavily influenced by our diet, lifestyle, and environment.
While genomic sequencing has provided valuable insights into the composition of the gut microbiome, metabolomics offers a unique and complementary perspective. By analyzing the metabolites produced by both our bodies and our gut microbes, metabolomics can provide real-time information about microbial activity and its impact on our health.
However, this level of functional insight is not achievable by sequencing alone. Bridging the gap between research capabilities and practical applications requires addressing various challenges, one of which is sample collection. Real-world application often requires frequent sampling to capture the dynamic nature of metabolic processes. This is particularly true when studying gut health, where metabolite profiles can change rapidly in response to diet, lifestyle, and other factors. Traditional sampling methods, especially for stool samples, can be inconvenient, costly, and often require specialized handling, making them impractical for frequent, long-term monitoring.

Solutions
To address this challenge, innovative sampling solutions have been introduced. One such approach is the S’Wipe technology, which aims to simplify the collection of stool samples for metabolomic analysis. This system integrates the sampling process into a user’s daily routine by utilizing a novel collection manifold that functions similarly to regular toilet paper.
The S’Wipe method preserves collected samples in an ethanol solution, which eliminates the need for refrigeration during storage and transportation. This approach potentially overcomes several limitations of existing stool sampling methods, including high costs, inconvenience, and the requirement for specialized equipment or facilities.
By simplifying the sample collection process, technologies like S-Wipe could enable larger-scale, longitudinal studies of the gut metabolome. Such studies are crucial for understanding the complex interactions between diet, the microbiome, and overall health.
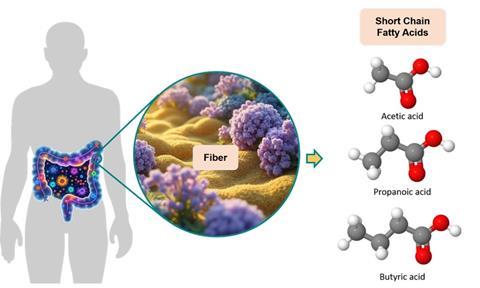
Tracking trends in gut metabolites can offer actionable insights into an individual’s gut health over time. These data can guide users towards personalized dietary interventions, such as specific fiber recommendations tailored to their unique gut microbiome composition and metabolic profile. For instance, if an individual’s SCFA levels are suboptimal, the analysis might suggest increasing consumption of particular types of dietary fibers known to promote SCFA production by their specific gut bacteria (Figure 1). This personalized approach could help optimize gut health and potentially improve overall well-being through targeted, data-driven dietary adjustments.
Further Reading:
Metabolomics Service Provider - Arome Science
Global chemical analysis of biology by mass spectrometry | Nature Reviews Chemistry
S-Wipe: stool sample collection for metabolomic gut health tracking | bioRxiv
Metabolomics: the apogee of the omics trilogy | Nature Reviews Molecular Cell Biology








No comments yet