As we strive towards a Net Zero society that is based on clean, green, and renewable technologies rather than fossil fuels, we are creating a new and intensifying reliance on another essential resource: metals. Meeting this rapidly increasing demand is a major challenge. Could microbes be the answer?
Metals are critical for green technologies such as electric vehicles, energy storage, wind turbines, solar photovoltaics, and electricity networks. In fact, electric vehicles require six times more metals and minerals than conventional vehicles (IEA 2021). All of these technologies will have to undergo rapid production expansion to achieve Net Zero targets, and as a result, the International Energy Authority has reported that mineral demand for clean energy technologies will quadruple by 2040. Accordingly, governments worldwide have published lists of minerals classified as “critical” due to their high economic importance and supply risks. The lists are growing longer over time; in the latest updates, the UK classified 34 minerals as critical, and the US and EU had as many as 50 and 51 critical materials respectively.
Meeting this rapidly increasing demand for critical metals is a major challenge, and could have substantial environmental consequences, as metals are mined, refined, used, and often discarded at the end-of-life in our current “take-make-waste” linear economy. In theory, metals can be recycled infinitely without loss of function, unlike other materials such as plastics and paper which deteriorate in quality when recycled, however recovery from end-of-life technology is becoming more challenging with the increasing range and complexity of product types. For example, the number of different metallic elements used in the production of electronic devices increased from 12 in the 1980s to as many as 60 in 2014 today. Separating these metals is a real challenge, and requires many energy and resource-intensive or polluting steps.
How does metal recycling actually work?
Traditional hydrometallurgical processes extract metals by dissolving them into solutions with strong mineral acids. Once in solution, the metals are separated and recovered through various techniques, including solvent extraction, electrowinning, and selective precipitation. However, these methods often come with significant downsides: they rely on costly and environmentally harmful organic solvents, consume large amounts of chemicals and water, and generate toxic acid and solvent waste.
Pyrometallurgy, an alternative high-temperature method, separates metals through physical or chemical transitions. While effective in some cases, it has its own challenges: high energy demands, limited selectivity for certain metals, and the generation of polluting gas emissions. Furthermore, valuable metals can become trapped in the solid waste slag, making them irretrievable.
These limitations make it difficult, uneconomical, or environmentally unsustainable to recover metals from complex waste materials, and innovative approaches are required to address these challenges and improve future metal recovery processes.
New microbial biotechnologies could change this outlook. Research has shown how we can harness the evolved selectivity of microbes, and their interactions with metals and minerals, to provide more sustainable solutions for extraction, separation, processing and even upcycling of technology-critical metals. Where many materials lose value with recycling, microbes can sometimes even provide new properties and functionalities to the materials they produce – enabling not just recycling, but “upcycling”. Applying tools for the engineering of biology can further expand the remit beyond their natural capabilities, creating a new host of microbial-derived technologies ready to solve global resource challenges.
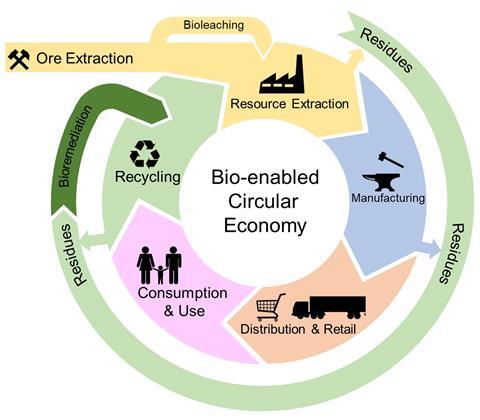
Bioleaching expands possibilities for sustainable metal extraction
The knowledge that microbes can play a significant role in the global metal supply chain is not new. Recovery of copper from acid-mine drainage was recorded in China as early as the 2nd century BCE, but it was not until many centuries later that the microbes responsible for creating these metal-rich waters were identified. Iron and sulfur-oxidising bacteria, adapted to low pH environments, such as Acidithiobacillus sp., Acidiphilium sp, Leptospirillum sp, Sulfobacillus sp, and archaea such as Ferroplasma sp., were discovered to play an essential role, and since the 1980s they have been exploited on an industrial scale within the mining industry in a process called bioleaching.
Bioleaching is currently applied around the world for the extraction of metals such as copper, gold, cobalt, and nickel, predominantly from sulfide-based minerals. At mines such as the Chambishi Copper Mine in Zambia, indigenous rock-associated microbes (lithotrophs) are screened, enriched adapted, and then inoculated onto open heaps of mined material. Acid irrigation of the heaps maintains suitable conditions for the microbial miners to get to work. Sulfur-oxidising microbes can directly oxidise metal sulfides, releasing them into solution. Additionally, microbial oxidation of iron sulfides in metal-bearing ores generates two powerful products: strongly oxidising ferric ions and sulfuric acid. Together, these agents can efficiently oxidise and leach metals such as copper, nickel, and cobalt, with low requirements for energy and other inputs. This enables the utilisation of minerals with metal concentrations too low to be economically mined using traditional chemical or pyrometallurgical approaches.
Bioleaching can also enable the mining industry to work towards a more circular economy, through the extraction of more value from the run-of-the-mine materials (tailings) remaining after traditional processing. In one Chilean copper mine, the introduction of bioleaching to process the tailings increased total copper recovery by 30%, while another bioleaching operation in Finland found a 32% reduction in CO2 emission compared to conventional processing. As high-grade ores begin to diminish worldwide, extraction from these lower-grade sources will become more and more important.

Utilisation of waste streams also has many benefits; reducing the need for environmentally damaging mining activities, and increasing the security of supply chains for countries with no exploitable reserves of critical resources themselves. In Finland, bioleaching was selected as the most suitable process for the recovery of battery-grade nickel and cobalt from arsenic-enriched industrial by-products. In this case, adapted consortia are contained in tanks rather than open heaps. The microbes leach nickel and cobalt and oxidise the environmentally toxic arsenic to a more stable form, allowing for safe disposal.
Bioleaching also enhances the recovery of other metals, such as gold, from previously unexploitable ores. “Biooxidation” of the iron-sulfide mineral matrix that entraps the valuable gold atoms is a commercialised pre-treatment technique that can greatly enhance extraction efficiencies: especially important when most gold sources contain just a few grams of gold per tonne.
However, bioleaching is still a slow process and can be vulnerable to instability or inhibition due to changes in feedstock compositions or environmental conditions. Current research is expanding the capabilities of bioleaching to recover a wider range of metals from different waste streams. Exploiting a wider range of microbes including organic-acid-producing fungi and heterotrophic bacteria could expand the range of metal sources that can be leached beyond sulfide ores.
Nature’s refinery: bio-separation of metals
Metal solubilisation is just one part of the challenge. Metals leached from waste sources are often part of a complex mixture of elements which can be challenging and energy-intensive to separate before they are ready for downstream application. Once again, attention is turning to the unique capabilities of the microbial world to develop new and sustainable solutions for metal separation and recovery.
When exposed to unusually high metal concentrations, some plants, fungi, and microorganisms have evolved detoxification mechanisms that remove these metals from solution, making them less able to interfere with cellular processes. Some of these organisms precipitate metals in the form of nanoparticles. Nanoparticles and nanomaterials specifically have one of their dimensions in the nano-range, which often confers unique properties in comparison to bulk materials, and have an expanding range of useful industrial and medical applications.
Another group of microbes has evolved to use metals in the environment to their advantage. For example, anaerobic sulfate-reducing bacteria such as Desulfovibrio sp. can use metals as the terminal electron acceptor to obtain energy from their respiratory chain, rather than molecular oxygen, reducing the metals in the process. These processes are controlled by a range of pathways, and can be metal specific, but are often highly condition-dependent. In the Horsfall group at the University of Edinburgh, our research exploits these microbes as re-usable platforms for efficient and selective metal recovery from waste leachates, enabling sustainable metal separation, recovery and even upcycling due to the novel material properties of nanoparticles produced during the process.
Bio-enabled recycling of Lithium-ion batteries
We focussed our expertise on one of the most pressing problems facing the global economy; the surge in demand for lithium-ion batteries (LIBs). Of all critical minerals, demand for lithium is forecast to increase the most, with a nine-fold increase expected by 2040, mainly driven by rapidly increasing numbers of LIB-containing electric vehicles hitting the roads. The projected mine supply is only expected to meet half of this. Furthermore, an efficient and sustainable recycling process for LIBs is yet to be developed, despite stockpiles of end-of-life LIBs exponentially increasing. The lithium-containing cathode can be separated from the battery and dissolved by biological or chemical leaching, releasing its composite metals into a solution (typically lithium, nickel, cobalt, manganese, and aluminium, in varying proportions depending on the cathode type). The next challenge is to separate the metals from this leachate to obtain battery-grade material for re-manufacture: it is this stage that traditional chemical methods have the most difficulties with.
Our research to tackle this problem resulted in a two-step process using bacteria to separate the metals from one another. We began with a high-throughput screen to find metal-tolerant microbes that were able to remove relevant metals from the battery cathode leachates. Through this work, the bacterium Shewanella oneidensis was shown to be an efficient remover of manganese in the form of manganese carbonate, under aerobic conditions. Carbonate production by this microbe also improves its tolerance to low pH, allowing it to be the first step in the bio-separation process of the acidic battery leachates.
In the second stage, the dissolved metals remaining after manganese removal are treated with the anaerobic metal-reducing bacterium Desulfovibrio alaskensis, which can remove cobalt as pure zero-valent or sulfide-containing nanoparticles and nickel as nickel sulfide nanoparticles. After treatment, we are left with a concentrated lithium solution. The manganese carbonate, cobalt and nickel bio-materials, and the lithium solution are potential precursors in the formation of new lithium-ion batteries, allowing the recovered materials to be re-processed back into the same technology from which they were derived.
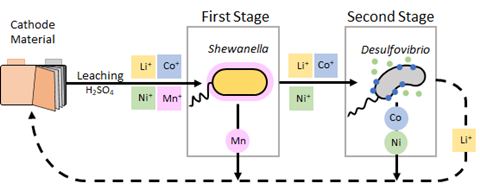
Since first developing the sequential bacterial treatment process, every stage has been optimised and scaled up, from lab scale to a 30-litre pilot scale bioreactor, enabling it to now approach feasibility for industrial application. The process can achieve high removal efficiencies of 96% for manganese, and >90% for nickel and cobalt.
Biologically produced nanoparticles have novel material properties
On a large scale, microbial recovery might not currently be viable as the sole technology for sustaining the global supply of bulk materials. Vast scales of fermentation would be necessary to meet the ever-increasing material demands. However, playing to the strength of the bacteria and recovering the metals in nanoparticle form can have unique benefits that could form a valuable part of the metal economy.
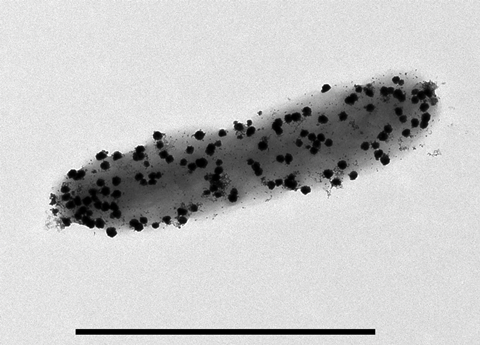
Nickel and cobalt nanoparticles recovered by this process have potential uses as electrocatalysts in Hydrogen Evolution Reactions or could be re-manufactured to new battery cathode material. Uniquely, this is the first time that microbial synthesis of zero-valent cobalt nanoparticles has been reported, potentially opening routes to new and exciting applications. Observations of these particles using transmission electron microscopy identify unique mesoporous structures in the nanoparticles produced by Desulfovibrio. Whilst research into the implications of these material properties is still underway in this case, there are plenty of examples of new and improved functionalities offered by biologically derived nanoparticles in comparison to their chemically synthesised counterparts.
Taking palladium nanoparticles as an example with already established and widespread application as catalysts in the chemical industry, we find that Desulfovibrio can produce palladium nanoparticles with high (>95%) yields. The biogenic (biologically produced) nanoparticles are more active than chemically synthesised equivalents and are remarkably good at the catalysis of reactions under Green Chemistry conditions. Nanoparticles attached to cell surfaces also offer unique opportunities to integrate non-natural chemical reactions with metabolic pathways, unlocking the sustainable and biocompatible synthesis of novel compounds that are currently inaccessible to engineered biological systems. We showed that biogenic palladium nanoparticles, when co-localised with an engineered hydrogen-producing E. coli strain, could catalyse one-pot cross-coupling (carbon-carbon bond formation) and hydrogenation reactions. These reactions were traditionally challenging for chemical systems to achieve and required hydrogen derived from natural gas. The addition of a vitamin-E derivative that self-assembles into small membrane-interacting compartments (micelles) allowed the co-localisation of the nanoparticles and substrates with the biologically produced hydrogen, enhancing the formation of the desired products.

Engineering biology opens new possibilities for future applications
Looking to the future, it is clear that the green transition will bring new demands for resources and new challenges for metal extraction, waste treatment, and recycling. Metal recovery processes may need to be adapted to other sources and waste streams, with varying compositions of metals and other contaminants. Biotechnology needs to be able to adapt at the same pace, and applying engineering principles to biology may be the solution to ensure that biotechnologies can continue to play a long-term role in a sustainable metal-based economy.
Many microbes with properties relevant to biotechnological applications are derived from extreme, waste-ridden environments, and are “non-model” organisms that do not currently have a wide range of tools or knowledge available for culturing and engineering in a lab or industrial setting. However, they often perform far better at metal recovery than standard laboratory model organisms such as E. coli, Bacillus, and Saccharomyces cerevisiae, due to the highly evolved and complex stress response pathways enabling their survival in these environments. Understanding the biological pathways underlying their abilities and expanding the genetic toolkits available for modifying these non-model organisms can create possibilities for future improvements.
Our group has investigated the response of a range of microbes to different metal treatments using proteomics. These studies aim to create a repository of knowledge, uncovering proteins and pathways that may not previously have been known to be associated with metal responses. For example, as proteomic data on Desulfovibrio highlighted a different biological response to platinum and palladium, we have begun to create strains with improved selectivity for either platinum or palladium nanoparticle formation, using the genetic tools we have developed for this species. The ability to target specific metals will be essential to improve bioseparation abilities and to adapt the process to different mixed-metal wastes.
We have also been able to tune the properties of biological nanoparticles in some cases. By overexpressing genes highlighted by proteomic studies such as those carried out on nickel/cobalt, or platinum/palladium, we have developed new strains that not only produce increased yields of metal nanoparticles targeting a specific metal but have also enabled us to modify the shapes and sizes of these particles. These bespoke nanomaterials have applications in industrially relevant reactions or as potential future materials with yet uncharacterised functions. Therefore, the knowledge arising from these studies can allow us to fine-tune the nanomaterial functionality toward the desired end application.
Conversely, once there is an increased understanding of the molecular and genetic features underlying metal tolerance and recovery processes, synthetic biology tools could be used to transfer these features to alternative host organisms. This could expand horizons by allowing metal recovery capabilities to be screened in different contexts, for example in organisms more amenable to large-scale fermentation.
In conclusion, a range of different approaches will be required to cope with future demands for critical metals. It will be essential to take a proactive rather than reactive approach, developing a range of technologies that can be part of a toolkit for a robust and sustainable circular economy for metals. Biotechnology can be one powerful tool in this toolkit, offering unique advantages compared to other more traditional technologies in terms of process sustainability and the possibilities for metal upcycling.







No comments yet